Fatal interstitial lung disease associated with icotinib
Introduction
In June 2011, icotinib (Conmana®, Zhejiang Beta pharmaceutical Co., Ltd., Hangzhou, China), a new Chinese made, orally active, small-molecule tyrosine kinase inhibitor (TKI) targeting the epidermal growth factor receptor (EGFR), was approved by China Food and Drug Administration (CFDA) and added to the treatment of non small cell lung cancer (NSCLC) in China. Similar to gefitinib and erlotinib, icotinib is an effective drug in recommended dose (125 mg, 3 times daily). However, its recommended dose is far from its tolerated dose. It shows potential superiority to erlotinib in a case report, in which a patient with lung adenocarcinoma after erlotinib failure responded to high dose icotinib (1). Additionally, it is a relatively safer drug compared with erlotinib (2). The most common adverse effects associated with icotinib are rash and diarrhea (3-8). The most serious, and maybe fatal, yet rare, adverse reaction of gefitinib and erlotinib is drug-associated interstitial lung disease (ILD), which has been often described (9-11). However, it has only been few documented in the clinical trials of icotinib. We present a fatal case of icotinib-associated ILD in a patient with lung adenocarcinoma and discuss it in the context of icotinib-associated ILD.
Case report
A 25-year-old female Chinese was diagnosed with stage IV lung adenocarcinoma with lymphangitis carcinomatosa and hydropericardium. Icotinib treatment was initiated once diagnosis of lung adenocarcinoma was made. Forty three days later, the patient died of hypoxic respiratory failure. Written consent was obtained from the patient’s parents for publication of this case report and any accompanying images.
The patient was healthy before the onset of illness. She had no history of smoking. She was admitted to other hospital for a complaint of dry cough and dyspnea. X-ray computed tomography (CT) of chest revealed multiple thickened bronchial wall and bronchiarctia in segment bronchus of left lung, mass shadows in apicoposterior segment and left posterior basal segment and diffuse nodular shadows in both lungs, with evidence for enlarged mediastinal, bilateral hilar and axillary lymph nodes (images were discarded by her parents after the patient was dead and were not available). There was irregular thickening of interlobular septal in bilateral lungs too. Diagnosis of stage IV lung adenocarcinoma with lymphangitis carcinomatosa was made when tranbronchial lung biopsy revealed adenocarcinoma. EGFR mutation in biopsy of lung tissue was tested negative by sequencing polymerase chain reaction. However, she had a poor World Health Organization (WHO) performance status (score 3). Chemotherapy was not reasonable and palliative treatment with icotinib (125 mg po, 3 times daily) was initiated. Ten days after treatment initiation, the patient developed a rash, a common adverse reaction of icotinib. Follow-up radiographs in our hospital 3 weeks after treatment initiation showed stable disease (SD) with no enlargement in the size of mass shadows and no increase in the number of nodular shadows.
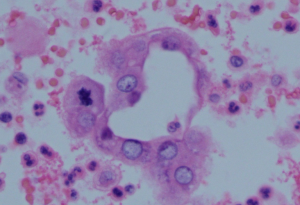
One month after icotinib treatment, while still on icotinib therapy, the patient was admitted to our hospital with continuous dry cough and rapid progressive dyspnea, denying lying down. Chest pain and haemoptysis was denied. Physical examination revealed decreased breath sounds in left lower lobe, some inspiratory crackles in right lower lobe and distant heart sounds. Oxygen saturation was 85% in room air and supplemental oxygen was given via nasal cannula. Leukocyte count was elevated with 15.92×109 cells/L. Blood cultures and sputum analysis initially was tested negative. Precursor of B-type natriuretic peptide was normal. Arterial blood gas analysis indicated presence of hypoxemic respiratory failure. Hence, pericardial effusion, left pleural effusion and lower respiratory tract infection was suspected. Ultrasound revealed massive pericardial effusion and left pleural effusion. Percutaneous pericardial drainage and chest catheter closed drainage was conducted. Pathological examination of pericardial fluid sediment revealed metastatic adenocarcinoma from lung (Figure 1). Intracavitary chemotherapy with etoposide (0.3 g) and cisplatin (100 mg) was administrated in the treatment of pericardial effusions. Antimicrobial therapy was pragmatically initiated with intravenous levofloxacin (500 mg once daily). Other medications included furosemide 20 mg, asmeton 2 tablets and selenious yeast tablets 100 µg (3 times daily).
However, clinical symptoms (including cough, dyspnea) and oxygen saturation was only partially improved and accompanied by fever 5 days after hospital admission. D-dimers were elevated with 5,467 ng/mL. Leukocyte count recovered to normal with 8.53×109 cells/L and procalcitonin was elevated with 0.81 ng/mL 1 week after admission. Multi-layer spiral CT angiography of pulmonary artery confirmed suspection of pulmonary artery embolism (Figure 2A). Multiple effusion shadows and ground-glass opacities were also found in bilateral lungs (Figure 2B). Hence, nadroparin (3,075 anti-Xa units twice daily) was initiated and warfarin was added later. Antibiotic was empirically changed to piperacillin-sulbactam (2,000/1,000 mg three times daily), and changed to meropenem (500 mg 3 times daily) together with intravenous voriconazole (200 mg twice daily) later.

Forty days after icotinib treatment, ILD was suspected when the patient still became increasingly dyspnoeic, despite of treatment of pericardial effusion, left pleural effusion and lower respiratory tract infection. The onset of continuous cough and dyspnea and characteristic newly emerged diffuse ground-glass opacities in bilateral lobe both supported the diagnosis of ILD (Figure 2B,C). The case scored 5 by using the Naranjo scale (12). Hence, it is probable that ILD was associated to icotinib. Icotinib was discontinued and intravenous corticosteroid was started (methylprednisolone 40 mg once daily, about 1 mg per kilogram) respectively. The condition of patient went worse even under the above medication. Forty-three days later, the patient died of hypoxic respiratory failure. Autopsy was not performed due to lack of permission from her parents.
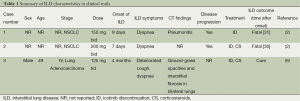
A PubMed and Google Scholar search was conducted using the keywords “pulmonary toxicity”, “ILD”, “icotinib” and “conmana®”. Similar Search was also conducted in a Chinese data-Wangfang Data. Relevant case reports and case-series were included and references of retrieved publications were screened for relevant literature. Until now, except for our case, a total of three cases of icotinib-associated ILD were reported in the clinical trial (Table 1). Two cases of ILD occurred within one month of icotinib treatment. Pathological examination showed that two cases of icotinib-associated ILD in phase I study of icotinib were accompanied with disease progression (4). One was considered disease-related and the other was considered both drug-related and disease-related. The third case appeared in phase IV study and diagnose of ILD was made based on ground-grass opacities and pulmonary fibrosis in CT scan, without evidence of biopsy (8). Other initial studies of icotinib leading to its approval and retrospective studies did not report pulmonary toxicity (3,6,7,13-15). There was no case report.
Full table
Discussion
The present case showed that icotinib may be associated with fatal ILD. To our knowledge, this is the first case report of fatal ILD associated with icotinib. Similar to other orally small molecule EGFR-TKIs, such as gefitinib and erlotinib, icotinib can cause or contribute significantly to the pulmonary toxicity, and thus this drug also must be considered among the EGFR-TKIs drugs which can cause fatal ILD. Respiratory symptoms should be carefully monitored for the first month period of icotinib treatment.
Severe ILD, including fatalities had been reported in clinical studies and case series with other EGFR-TKIs (16-18). For gefitinib-treated patients, FDA reported that incidence of ILD was 1% worldwide, of which more than 30% were fatal (10). The incidence of ILD varied with countries. It was higher in Japan (2%) than in the United States (0.3%) (19). In the FDA approval report, overall incidence of ILD in erlotinib-treated patients was about 0.8%, which was similar to patients in the placebo group (11). Overall incidence of ILD in icotinib treated patients was still unknown. However, results of another phase IV study in which more than 10,000 patients were enrolled are round the corner.
Thus far, no risk factors for the development of icotinib-associated ILD have been described. However, several risk factors were considered to be related to gefitinib-associated ILD, including older age, poor World Health Organization (WHO) performance status (≥2), smoking, recent NSCLC diagnosis, reduced normal lung on CT scan, preexisting chronic ILD, concurrent cardiac disease and Japanese ethnicity (20). The higher incidence of ILD in Japanese patients was considered to be contributed to the larger percentage of patients with EGFR mutations and clinical responses to gefitinib (21). Other studies have suggested recent radiation therapy and chemotherapy as risk factors for ILD (22). Most of ILD occur within 3-7 weeks after initiation of gefitinib and one third of cases are fatal. Risk factors of erlotinib-associated ILD were similar to gefitinib, with a median of 47 days after initiation therapy (11,17,23). Several of these risk factors were present in our case, including recent NSCLC diagnosis (1 month), poor WHO performance status, reduced normal lung on CT scan (diffuse nodular shadows in bilateral lungs, left pleural effusion, multiple pulmonary artery embolisms) and concurrent cardiac disease (massive pericardial effusion). Including our cases, disease progression was confirmed by pathological examination in three out of four cases of icotinib-associated ILD and it maybe consider as one of the risk factors of icotinib-associated ILD. Overall, three out of four cases of ILD occurred within one month of initiation of icotinib. Two cases of ILD reported in clinical trials of icotinib were treated with chemotherapy before initiation of icotinib. Whether risk factors of icotinib-associated ILD have its uniqueness or are similar to gefitinib and erlotinib, it remains to be investigated.
However, the mechanism for developing icotinib-associated ILD is still unknown. Phase I study of icotinib demonstrated that there was no obvious relationship between advert effects/severe advert effects and dose (3,4,24). Pharmocology and clinical evaluation revealed that the therapeutic window for icotinib-defined as the tolerable and effective dose range was 100-625 mg three times per day (24). Dose of icotinib in all of four cases of icotinib-associated ILD ranged 300-400 mg per day, which was lower than highest tolerable and effective dose. Hence, icotinib-associated ILD may seem unlikely to be dose dependent. Considering the similarities of EGFR-TKIs in chemical structure and pharmacological effects, it is likely that the mechanism for development of icotinib-associated ILD is comparable with gefitinib and erlotinib. However, whether icotinib can induce pulmonary toxicity by a mechanism related to its unique properties or similar to other EGFR-TKIs (gefitinib and erlotinib), it remains to be studied.
It is worth to note that, three out of four cases of icotinib-associated ILD were fatal. Fatality of ILD induced by icotinib was higher than that of gefitinib and erlotinib, although all three fatal cases were accompanied by disease progression. In view of poor information concerning risk factors and mechanism of icotinib-associated ILD, prevention and treatment of icotinib-associated ILD should borrow from gefitinib and erlotinib associated ILD. Medical history, including respiratory symptoms, signs, chemotherapy or radiation, etc., should be well reviewed prior to the prescription of medication. Icotinib-associated ILD should be highly suspected once respiratory symptoms (especially dry cough and dyspnea) worsen. Discontinuation of medication, initiation of high dose corticosteroids and supportive therapy with oxygen therapy or mechanical ventilation was reported in case series as the only useful interventions in the treatment of gefitinib and erlotinib associated ILD (9,25,26).
Limitation of our report was that cytomegalovirus CMV infection and pneumocystis jiroveci pneumonia could not be ruled out. Serological test for CMV infection was not performed. Because the condition of the patient was going bad rapidly, bronchoscopy could not be performed and sputum from deep position or bronchoalveolar lavage was not available for bacterial and fungal culture or detection of CMV and pneumocystis jiroveci. Additionally, biopsy of lung also could not be done to confirm pathology of ILD.
Acknowledgements
The authors thank Jin Zhao, Pathological Department of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou Institute of Respiratory Medicine, the State Key Laboratory of Respiratory Disease (Guangzhou Medical College) for supply of pathological images.
Authors Z.YQ and Z. JX participated in the PubMed and Google Scholar search for cases of icotinib associated ILD. All authors participated in the discussion of the data and contributed to the drafting of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Guan Y, Zhao H, Meng J, et al. Dramatic response to high-dose icotinib in a lung adenocarcinoma patient after erlotinib failure. Lung Cancer 2014;83:305-7. [PubMed]
- Liang W, Wu X, Fang W, et al. Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations. PLoS One 2014;9:e85245. [PubMed]
- Zhao Q, Shentu J, Xu N, et al. Phase I study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung Cancer 2011;73:195-202. [PubMed]
- Wang HP, Zhang L, Wang YX, et al. Phase I trial of icotinib, a novel epidermal growth factor receptor tyrosine kinase inhibitor, in Chinese patients with non-small cell lung cancer. Chin Med J (Engl) 2011;124:1933. [PubMed]
- Zhao Q, Zhou J, Shentu J, et al. A phase I/IIa study of icotinib hydrochloride, a novel oral EGFR-TKI, to evaluate its safety, tolerance, and preliminary efficacy in advanced NSCLC patients in China. J Clin Oncol 2010;28.
- Lv C, Ma Y, Feng Q, et al. A pilot study: sequential gemcitabine/cisplatin and icotinib as induction therapy for stage IIB to IIIA non-small-cell lung adenocarcinoma. World J Surg Oncol 2013;11:96. [PubMed]
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953-61. [PubMed]
- Tan FL, Wang YX, Ding LM, et al. Safety and Efficacy of Icotinib Hydrochloride for Non-small Cell Lung Cancer (NSCLC) Patient in A Large Population. Chinese Journal of Drug Evaluation 2012;29:30-4.
- Barber NA, Ganti AK. Pulmonary toxicities from targeted therapies: a review. Target Oncol 2011;6:235-43. [PubMed]
- Cohen MH, Williams GA, Sridhara R, et al. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 2003;8:303-6. [PubMed]
- Cohen MH, Johnson JR, Chen YF, et al. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist 2005;10:461-6. [PubMed]
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45. [PubMed]
- Gu A, Shi C, Xiong L, et al. Efficacy and safety evaluation of icotinib in patients with advanced non-small cell lung cancer. Chin J Cancer Res 2013;25:90-4. [PubMed]
- Nong J, Qin N, Wang J, et al. Clinical effects for patients with recurrent advanced non-small cell lung cancer treated with icotinib hydrochloride. Zhongguo Fei Ai Za Zhi 2013;16:240-5. [PubMed]
- Li X, Yang XJ, Sun YF, et al. Clinical observation of icotinib hydrochloride for patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2012;34:627-31. [PubMed]
- Jackman DM, Yeap BY, Lindeman NI, et al. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol 2007;25:760-6. [PubMed]
- Makris D, Scherpereel A, Copin MC, et al. Fatal interstitial lung disease associated with oral erlotinib therapy for lung cancer. BMC Cancer 2007;7:150. [PubMed]
- Peerzada MM, Spiro TP, Daw HA. Pulmonary toxicities of tyrosine kinase inhibitors. Clin Adv Hematol Oncol 2011;9:824-36. [PubMed]
- Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer 2004;91:S18-23. [PubMed]
- Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008;177:1348-57. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Inoue A, Saijo Y, Maemondo M, et al. Severe acute interstitial pneumonia and gefitinib. Lancet 2003;361:137-9. [PubMed]
- Liu V, White DA, Zakowski MF, et al. Pulmonary toxicity associated with erlotinib. Chest 2007;132:1042-4. [PubMed]
- Tan F, Zhang L, Zhao Q. Pharmocology and clinical evaluation of icotinib hydrochloride. Chin J New Drugs 2009;18:1-4.
- Kuo LC, Lin PC, Wang KF, et al. Successful treatment of gefitinib-induced acute interstitial pneumonitis with high-dose corticosteroid: a case report and literature review. Med Oncol 2011;28:79-82. [PubMed]
- ter Heine R, van den Bosch RT, Schaefer-Prokop CM, et al. Fatal interstitial lung disease associated with high erlotinib and metabolite levels. A case report and a review of the literature. Lung Cancer 2012;75:391-7. [PubMed]


