The protective effects of glutamine in a rat model of ventilator-induced lung injury
Introduction
Acute respiratory distress syndrome (ARDS) is a leading cause of death in critically ill patients (1,2). ARDS is characterized by leukocyte recruitment and increased permeability in the pulmonary capillaries and epithelial barrier resulting in lung edema and tissue hypoxia. Almost all patients with ARDS require mechanical ventilation (MV) for life support (2). However, the utilization of MV may cause alveolar overdistension or shear forces generated during the repetitive opening and closing of the lung units, resulting in atelectasis that leads to ventilator-induced lung injury (VILI) (2). The VILI is frequently associated with multiple distal organ dysfunction as a result of biotrauma (3). The activation of nuclear factor (NF)-κB with the subsequent gene transcription of inflammatory mediators has been previously documented in the context of VILI (4,5). Although the use of low tidal volume has been shown to improve the clinical outcome of certain populations of patients with ARDS, the overall mortality rate of patients with ARDS is still high (6). Furthermore, the application of MV tends towards less “controlled” in the ICU than in the operating room because more patients receiving ventilation are conscious during their ICU stay, making designated low tidal volume ventilation challenging (7-9). There is, therefore, a need to identify therapeutic approaches in addition to the use of protective MV strategies in the management of ARDS.
Glutamine (GLN) is a conditional essential amino acid that serves as an important energy source for cell proliferation (10). GLN is an essential component for numerous metabolic functions including acid-base homeostasis, gluconeogenesis, nitrogen transport and the synthesis of proteins and nucleic acids (11). GLN has been demonstrated to protect the lung from injury in animal models of ischemia-reperfusion injury, endotoxemia, hyperoxia, smoke inhalation and sepsis (12-17). GLN has been shown to exert immunomodulating properties that attenuate the production of cytokines and chemokines in response to oxidative stress (18) and in animal models of endotoxin-induced lung injury (16,19,20). The beneficial effects of GLN are believed to be associated with the attenuation of NF-κB nuclear translocation or activation, the enhancement of heat shock protein expression (12,21), and the precursor property for glutathione (GSH) synthesis (16,22,23). However, the effects of treatment with GLN are inconclusive in critically ill patients (11,24-27), which might be due to the complicated underlying diseases and the timing of the therapeutic intervention. Because physicians know exactly when MV is applied at bedside, we thus test the hypothesis that the administration of GLN is beneficial to the attenuation of VILI via its anti-inflammatory effects when administered at the onset of MV following acute lung injury.
Materials and methods
Animal preparation
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Care and Use Committee of Chi-Mei Hospital (Permit Number: CMHFR10212). All surgery was performed under general anesthesia, and all efforts were made to minimize suffering. Male Sprague-Dawley rats (300-400 g) were anesthetized by the intraperitoneal injection of urethane (2 mg/kg, Sigma, St. Louis, MO, USA). Endotoxin (Escherichia coli O55:B5, Sigma) was administered intratracheally at 0.1 mg/kg. The animals were allowed to recover from anesthesia and returned to their cages with access to food and water for 24 h.
The animals were then anesthetized by the intraperitoneal injection of urethane. An intravenous cannula was inserted and kept in the tail vein for the maintenance of anesthesia by a continuous infusion of ketamine (15 mg/kg/h), xylazine (3 mg/kg/h), and pancuronium (0.35 mg/kg/h) and infusion of lactated Ringer’s solution. A tracheostomy was performed, and a 14-gauge cannula (Angiocath IV, 2.1×48 mm, Becton Dickinson Infusion Therapy Systems, Sandy, UT) was inserted into the trachea. The rats were ventilated with a Servo 300 ventilator (Siemens, Sweden) using a tidal volume (Vt) of 6 mL/kg, positive end-expiratory pressure (PEEP) of 5 cmH2O, and respiratory rate of 50 breaths/min with a fraction of inspired oxygen (FiO2) of 0.4. The right carotid artery was cannulated (Angiocath IV Catheter; 24-gauge) to monitor the mean arterial pressure (MAP) and to collect samples for blood gas measurements.
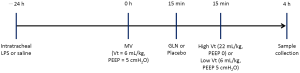
The animals were stabilized for 30 min after the surgical preparation and assigned to receive MV for 4 h randomly at either low or high Vt. The low Vt groups were ventilated with a Vt of 6 mL/kg and PEEP of 5 cmH2O at a rate of 45-55 breaths/min. The high Vt groups were ventilated with a Vt of 22 mL/kg and zero PEEP at a respiratory rate of 16-18 breaths/min. The FiO2 remained as 0.4. GLN (20% Dipeptiven, Fresenius Kabi, Germany) containing 20 g of N(2)-L-alanyl-L-glutamine at a bolus of 0.75 g/kg (3.75 mL/kg) or lactated Ringer’s solution as placebo, was administered i.v. in a blind fashion 15 min prior to the randomization of the MV strategies.
Additional groups of naive control and LPS alone without ventilation served as time-matched controls (Figure 1). Thus, six groups of ten animals each were studied: naive control animals without LPS and ventilator (Naive), LPS-challenged animals receiving no MV (Control), LPS-challenged animals receiving MV at low Vt (LV), LPS-challenged animals receiving MV at high Vt (HV), LPS-challenged animals receiving MV at low Vt and treated with GLN (LVG), and LPS-challenged animals receiving MV at high Vt and treated with GLN (HVG).
Measurements
Lung elastance was calculated hourly using the formula of (Plateau Pressure—PEEP)/Vt during the study. Blood gases were measured at the beginning of the randomization and hourly thereafter.
The rats were sacrificed by sodium pentobarbital overdose. The lungs were excised via a midline sternotomy, and the static pressure-volume curves were constructed by the manual injection of 0.5 to 1 cm3 aliquots of air in a stepwise manner starting at atmospheric pressure and continuing until achieving an airway pressure of 30 cmH2O. This procedure was followed by deflation using a similar stepwise approach. Volumes were maintained at each step of air inflation or deflation for 6 seconds.
The left lung was lavaged (bronchoalveolar lavage, BAL) for the assessment of cell differentiation and measurement of cytokines. The right upper lungs were used to measure the wet-to-dry (W/D) lung weight ratio. The measurement of cytokines (IL-1β, IL-6, IL-10, CXCL1 and TNF-α) in the plasma and BAL fluid was performed in a blinded fashion by technicians using rat-specific DuoSet ELISA kits (R & D Systems, Minneapolis, MN, USA).
The rest of the right lungs were used for the histological analysis and assessment of lung injury. The lung injury score was evaluated by a pathologist (CFL) who was blinded to the experimental groups, based on the methods described previously, including neutrophil counts in the alveoli and interstitial space, hyaline membrane, proteinaceous debris filling the airspaces, and alveolar septal thickening (28). Five regions in each lung histology slide were examined. The resulting injury score was represented by a continuous value between zero and one.
Statistical analysis
Data are reported as the means ± standard error and were analyzed by SPSS 13.0 (SPSS Inc. Chicago, IL, USA) using one-way analysis of variance, followed by Bonferroni’s multiple comparisons. Two-sided analysis was performed and the significance level was considered to be P<0.05.
Results
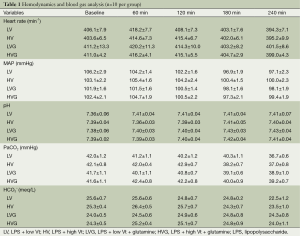
The mean body weight (355, 351, 359, 363, 357 and 357 g in the Naive, Control, LV, HV, LVG and HVG group, respectively) and length (25.8, 25.7, 26.0, 26.4, 25.9 and 25.8 cm, respectively) was similar in all animals studied. The hemodynamics, including MAP and heart rate, were also similar at baseline and did not differ in all animals during the study (Table 1). The amount of fluid infused was identical (≈2.5 mL lactated Ringer’s solution) in all rats during the study.
Full table
The values of blood gas were similar at baseline in all animals, but the oxygen indices, including PaO2 and SaO2, were significantly lower in the HV group at 120 min and onwards (respectively, 149.1±7.6 mmHg and 97.7±0.3% at 120 min, 118.7±11.1 mmHg and 92.6±1.8% at 180 min, 102.1±10.8 mmHg and 89.6±2.0% at 240 min), compared with the other groups (All P<0.001) (Figure 2A,B).
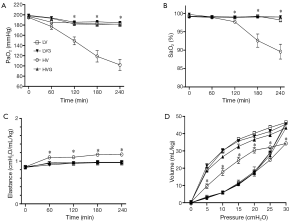
The lung elastance levels were similar at baseline but increased significantly in the HV group compared with the other groups at 60 min after randomization (Figure 2C). Similarly, the static pressure-volume curves showed the worst compliance in the HV group compared with the other groups at the end of the study (Figure 2D).
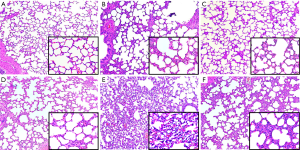
The representative lung histology showed aggravated alveolar collapse and perivascular and peribronchial edema associated with neutrophil infiltration in the HV group. The administration of GLN significantly attenuated the lung injury (Figure 3).
The lung W/D ratio and the lung injury score were significantly higher in the HV group than in the other groups (all P<0.001) (Table 2). The treatment with GLN resulted in a significant reduction in lung injury after MV with a high Vt (Table 2).
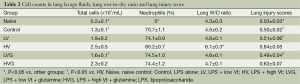
Full table
The total cell count in BAL fluid was somewhat higher in the HV group [(2.5±0.5)×107/mL], but the difference was not significant compared with the HVG group [(2.3±0.2)×107/mL, P=0.290] and LV group [(1.9±0.2)×107/mL, P=0.088] (Table 2). The total cell count was significantly lower in the LVG [(1.6±0.1)×107/mL, P=0.011] and naive (0.2±0.1×107/mL, P<0.001) groups than in the HV group (Table 2). The neutrophil count was significantly higher in the HV group than in the other groups (all P<0.001) (Table 2).
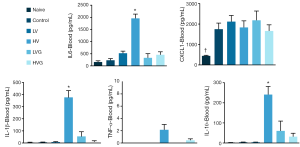
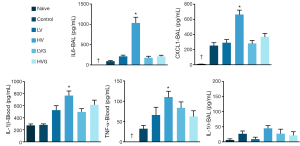
The plasma levels of IL-1β, IL-6 and IL-10 were higher in the HV group than in the other groups (Figure 4). The levels of IL-1β, IL-6, CXCL1 and TNF-α in the BAL fluids were higher in the HV group than in the other groups (Figure 5).
Discussion
In the present study, we demonstrated that the administration of GLN protected the lung from the two-hit injury by the attenuation of inflammatory responses, including neutrophil infiltration and cytokine storm. The animals treated with GLN showed better lung mechanics and lung morphology under high Vt MV compared with the placebo group. These results suggest a beneficial effect of the administration of GLN in the context of VILI.
Inflammation and oxidative stress play important roles in the pathogenesis of VILI (3). The release of TNF-α, IL-1β, IL-6 and the CXC chemokine family is associated with neutrophil recruitment (29) and correlated with the severity and mortality of ARDS (30). CXCL1, the homolog of human IL-8 in rats, plays a pivotal role as a chemotactic factor for neutrophil infiltration (31). IL-8 has been shown to be released by lung epithelial cells in response to mechanical stress (32). Previous studies reported that the administration of GLN can reduce IL-1β, IL-8 and TNF-α in LPS-induced lung injury (19), diminish IL-6 in abdominal sepsis (33,34), and inhibit IL-6, IL-8 and TNF-α production in human monocytes stimulated with LPS (35). The anti-inflammatory effects of GLN observed in other models used in previous studies were confirmed in our model of VILI in the present study.
Neutrophil activation is largely responsible for not only the production of cytokines and chemokines but also oxidative bursts and release of proteolytic enzymes, all contributing to lung injury (6,36,37). In particular, intracellular oxidative stress leads to vascular barrier disruption and pulmonary edema (38,39). Our results observed in the ARDS/VILI model are consistent with those demonstrated in other models, in which the administration of GLN led to significantly reduced neutrophil recruitment in the lung (33,40). The two most important mechanisms by which GLN is protective include its antioxidant effect through the preservation of GSH and the induction of heat shock protein 70 via the O-linked glycosylation-dependent activation of hexosamine biosynthesis (41,42). This heat shock protein is known to enhance cell survival and attenuate the systemic inflammatory response in the setting of lung injury (43). In particular, the enhanced levels of tissue GSH and heat shock proteins may be partially responsible for the attenuated cytokine responses (44) and activation of NF-κB (23,45).
The importance of GLN may have been overlooked in ARDS. Patients with critical illness have been reported to be at high risk of GLN depletion, which might contribute to the development of ARDS (46). In fact, GLN deficiency may have caused failure of the host defense mechanism, delayed wound healing, increased epithelial permeability and bacterial translocation (22). In addition, GLN–enriched parenteral nutrition has been shown to augment glucose use (47) and decrease insulin resistance (48). In experimental models, GLN supplementation was protective against lung injury induced by ischemia-reperfusion, endotoxins, hyperoxia, smoke inhalation and sepsis (12-17).
In clinical settings, a recent trial and a meta-analysis did not show significant beneficial effects in critically ill patients treated with GLN (24,25). However, several randomized, controlled clinical trials have reported that supplementation with GLN may reduce the occurrence of infections, length of the hospital stay and mortality rate in critically ill patients (11,26) and may improve the survival rate in burn patients with a Gram-negative bacterial infection (27). Current guidance on nutritional support by the European Societies of Parenteral and Enteral Nutrition recommends GLN supplementation at the grade A level for critically ill patients (49).
In the context of VILI, we employed the two-hit model to reproduce the clinical features of ARDS. Our model used in the present study is different from previous studies testing the effects of GLN in single injury models (16,33,40). For example, (I) we used a relatively low dose of LPS to prime the lung followed by MV to induce VILI, as observed in clinical situations of ARDS. The previous studies used a single hit with either a high dose of LPS or severe sepsis to study the effects of GLN in infection (16,33). Therefore, our study focused on the effects of GLN on VILI. (II) We administered GLN after LPS instillation, but GLN was given as a pre-treatment prior to LPS induction in a previous study (19). Our model addressed the effects of GLN after an inflammatory response has been established. (III) We administered GLN 30 min before the randomization of the MV strategies in the attempt to attenuate VILI, which is appropriate as the exact time when MV would be applied at bedside is known. This treatment approach would be very relevant to bedside management for ARDS/VILI.
In summary, the beneficial effects of GLN in our study can be explained by several potential mechanisms: (I) the attenuation of neutrophil infiltration in the lung, which is a significant factor in the pathogenesis of ARDS; (II) the reduction of biotrauma, including the cytokine responses (21,34); (III) the preservation of tissue GSH levels that maintain tissue antioxidant capacity by the administration of GLN (16,23,45); and (IV) the up-regulation of heat shock protein 70 following the treatment with GLN (12).
A major limitation of the study is that we were unable to measure the plasma levels of GLN and oxidative stress. Other investigators have previously demonstrated that intracellular GLN depletion in muscle occurred early and remained low during ICU stay (50) and that the low plasma level of GLN was associated with the high mortality (51). Therefore, the beneficial effects of GLN might have been overlooked in the context of VILI. Further research in the field is required to confirm our findings for a potential novel therapeutic target in VILI. The other limitation is that we used only one dose of GLN (0.75 mg/kg), which was similar to the dose used in most previous studies (12-14,16,19). Because the possibility that multiple doses, continuous infusion and/or the enteral route could yield better histological results is not fully excluded, the sensitivity and specificity of the effect cannot be ascertained.
Conclusions
In the present study, we demonstrate that the treatment with GLN administered immediately prior to MV may be beneficial in reducing VILI during ARDS.
Acknowledgements
Authors’ contributions: Conceived and designed the experiments: CMC and HZ. Performed the experiments: CMC. Analyzed the data: CMC, KCC and CFL. Contributed reagents/materials/analysis tools: CMC and KCC. Wrote the paper: CMC and HZ.
Disclosure: The authors declare no conflict of interest.
References
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [PubMed]
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685-93. [PubMed]
- Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 1997;99:944-52. [PubMed]
- Held HD, Boettcher S, Hamann L, et al. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappaB and is blocked by steroids. Am J Respir Crit Care Med 2001;163:711-6. [PubMed]
- Ding N, Wang F, Xiao H, et al. Mechanical ventilation enhances HMGB1 expression in an LPS-induced lung injury model. PLoS One 2013;8:e74633. [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [PubMed]
- Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 2010;375:475-80. [PubMed]
- Ferguson ND. Low tidal volumes for all? JAMA 2012;308:1689-90. [PubMed]
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 2004;32:1817-24. [PubMed]
- Castell L. Glutamine supplementation in vitro and in vivo, in exercise and in immunodepression. Sports Med 2003;33:323-45. [PubMed]
- Oliveira GP, Dias CM, Pelosi P, et al. Understanding the mechanisms of glutamine action in critically ill patients. An Acad Bras Cienc 2010;82:417-30. [PubMed]
- Singleton KD, Serkova N, Beckey VE, et al. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med 2005;33:1206-13. [PubMed]
- Singleton KD, Serkova N, Banerjee A, et al. Glutamine attenuates endotoxin-induced lung metabolic dysfunction: potential role of enhanced heat shock protein 70. Nutrition 2005;21:214-23. [PubMed]
- Perng WC, Huang KL, Li MH, et al. Glutamine attenuates hyperoxia-induced acute lung injury in mice. Clin Exp Pharmacol Physiol 2010;37:56-61. [PubMed]
- Peng CK, Huang KL, Wu CP, et al. Glutamine protects ischemia-reperfusion induced acute lung injury in isolated rat lungs. Pulm Pharmacol Ther 2011;24:153-61. [PubMed]
- Zhang F, Wang X, Pan L, et al. Glutamine attenuates lipopolysaccharide-induced acute lung injury. Nutrition 2009;25:692-8. [PubMed]
- Li W, Qiu X, Wang J, et al. The therapeutic efficacy of glutamine for rats with smoking inhalation injury. Int Immunopharmacol 2013;16:248-53. [PubMed]
- Coëffier M, Miralles-Barrachina O, Le Pessot F, et al. Influence of glutamine on cytokine production by human gut in vitro. Cytokine 2001;13:148-54. [PubMed]
- Liu JC, Wang HT, Wang W. Protective effects of alanyl-glutamine on acute lung injury induced by lipopolysaccharide in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2008;33:1095-100. [PubMed]
- Hou YC, Pai MH, Chiu WC, et al. Effects of dietary glutamine supplementation on lung injury induced by lipopolysaccharide administration. Am J Physiol Lung Cell Mol Physiol 2009;296:L288-95. [PubMed]
- Singleton KD, Beckey VE, Wischmeyer PE. Glutamine prevents activation of NF-kappaB and stress kinase pathways, attenuates inflammatory cytokine release, and prevents acute respiratory distress syndrome (ARDS) following sepsis. Shock 2005;24:583-9. [PubMed]
- Wernerman J. Clinical use of glutamine supplementation. J Nutr 2008;138:2040S-2044S. [PubMed]
- Zhang F, Wang X, Wang W, et al. Glutamine reduces TNF-alpha by enhancing glutathione synthesis in lipopolysaccharide-stimulated alveolar epithelial cells of rats. Inflammation 2008;31:344-50. [PubMed]
- Bollhalder L, Pfeil AM, Tomonaga Y, et al. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr 2013;32:213-23. [PubMed]
- Andrews PJ, Avenell A, Noble DW, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;342:d1542.
- Wischmeyer PE. Glutamine: role in critical illness and ongoing clinical trials. Curr Opin Gastroenterol 2008;24:190-7. [PubMed]
- Lin JJ, Chung XJ, Yang CY, et al. A meta-analysis of trials using the intention to treat principle for glutamine supplementation in critically ill patients with burn. Burns 2013;39:565-70. [PubMed]
- Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011;44:725-38. [PubMed]
- Williams AE, Chambers RC. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol 2014;306:L217-30. [PubMed]
- Moldoveanu B, Otmishi P, Jani P, et al. Inflammatory mechanisms in the lung. J Inflamm Res 2009;2:1-11. [PubMed]
- Levitt JE, Gould MK, Ware LB, et al. The pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med 2009;24:151-67. [PubMed]
- Vlahakis NE, Schroeder MA, Limper AH, et al. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol 1999;277:L167-73. [PubMed]
- Oliveira GP, Oliveira MB, Santos RS, et al. Intravenous glutamine decreases lung and distal organ injury in an experimental model of abdominal sepsis. Crit Care 2009;13:R74. [PubMed]
- Yeh CL, Hsu CS, Yeh SL, et al. Dietary glutamine supplementation modulates Th1/Th2 cytokine and interleukin-6 expressions in septic mice. Cytokine 2005;31:329-34. [PubMed]
- Raspé C, Czeslick E, Weimann A, et al. Glutamine and alanine-induced differential expression of intracellular IL-6, IL-8, and TNF-α in LPS-stimulated monocytes in human whole-blood. Cytokine 2013;62:52-7. [PubMed]
- Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med 2011;17:293-307. [PubMed]
- Zhang H, Downey GP, Suter PM, et al. Conventional mechanical ventilation is associated with bronchoalveolar lavage-induced activation of polymorphonuclear leukocytes: a possible mechanism to explain the systemic consequences of ventilator-induced lung injury in patients with ARDS. Anesthesiology 2002;97:1426-33. [PubMed]
- Davidovich N, DiPaolo BC, Lawrence GG, et al. Cyclic stretch-induced oxidative stress increases pulmonary alveolar epithelial permeability. Am J Respir Cell Mol Biol 2013;49:156-64. [PubMed]
- Reddy SP, Hassoun PM, Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal 2007;9:2003-12. [PubMed]
- Murphy CG, Chen G, Winter DC, et al. Glutamine preconditioning protects against tourniquet-induced local and distant organ injury in a rodent ischemia-reperfusion model. Acta Orthop 2007;78:559-66. [PubMed]
- Hamiel CR, Pinto S, Hau A, et al. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am J Physiol Cell Physiol 2009;297:C1509-19. [PubMed]
- Singleton KD, Wischmeyer PE. Glutamine induces heat shock protein expression via O-glycosylation and phosphorylation of HSF-1 and Sp1. JPEN J Parenter Enteral Nutr 2008;32:371-6. [PubMed]
- Wheeler DS, Wong HR. Heat shock response and acute lung injury. Free Radic Biol Med 2007;42:1-14. [PubMed]
- Ribeiro SP, Villar J, Downey GP, et al. Effects of the stress response in septic rats and LPS-stimulated alveolar macrophages: evidence for TNF-alpha posttranslational regulation. Am J Respir Crit Care Med 1996;154:1843-50. [PubMed]
- Belmonte L, Coëffier M, Le Pessot F, et al. Effects of glutamine supplementation on gut barrier, glutathione content and acute phase response in malnourished rats during inflammatory shock. World J Gastroenterol 2007;13:2833-40. [PubMed]
- Planas M, Schwartz S, Arbós MA, et al. Plasma glutamine levels in septic patients. JPEN J Parenter Enteral Nutr 1993;17:299-300. [PubMed]
- Déchelotte P, Hasselmann M, Cynober L, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med 2006;34:598-604. [PubMed]
- Bakalar B, Duska F, Pachl J, et al. Parenterally administered dipeptide alanyl-glutamine prevents worsening of insulin sensitivity in multiple-trauma patients. Crit Care Med 2006;34:381-6. [PubMed]
- Singer P, Berger MM, Van den Berghe G, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr 2009;28:387-400. [PubMed]
- Rotstein OD. Modeling the two-hit hypothesis for evaluating strategies to prevent organ injury after shock/resuscitation. J Trauma 2003;54:S203-6. [PubMed]
- Oudemans-van Straaten HM, Bosman RJ, Treskes M, et al. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 2001;27:84-90. [PubMed]