Advanced therapies for COPD—What’s on the horizon? Progress in lung volume reduction and lung transplantation
Introduction
Chronic obstructive pulmonary disease (COPD) is the 4th leading cause of mortality also conferring significant adverse impact on the quality of life for millions of people world wide (1). Goals of treatment are avoidance of disease progression by cessation of noxious particulate exposure, improving exercise capacity by participation in pulmonary rehabilitation, prescription of pharmacotherapy and reducing exacerbation rate (2). Despite these measures a large proportion of patients continue to experience functional impairment and diminished quality of life with consequential economic and social burden (3). This article will explore advanced therapies and surgical interventions for patients who remain impaired despite optimal medical care. The mainstay of treatment options are:
- Lung volume reduction surgery (LVRS);
- Lung transplantation.
Although yet to be integrated into widespread clinical practise, bronchoscopic methods of lung volume reduction (LVR) are currently being developed. These potentially represent a less invasive, more accessible treatment option for advanced emphysema.
Lung volume reduction (LVR) practises
Physiological basis for LVR
Airway obstruction and emphysema both cause hyperinflation leading to alterations in both lung and chest wall mechanics (4). The combination of impaired gas exchange, unfavourable lung mechanics at high volume and respiratory muscle inefficiency (due to the respiratory muscles being placed at a mechanical disadvantage) lead to a substantial (and unsustainable) increased work of breathing. Loss of elastic recoil and dynamic airway closure during expiration cause increases in intrinsic PEEP and gas trapping. In these circumstances greater respiratory effort is required to overcome these loads to achieve similar alveolar ventilation. The resulting hyperinflation further exacerbates the problem by reducing respiratory muscle efficiency through diaphragmatic flattening. These physiological alterations result in symptoms of dyspnoea and reduction in exercise capacity. LVR techniques aim to improve respiratory mechanics by resecting, collapsing or obliterating areas of diseased lung making a poor contribution to gaseous exchange. The remaining lung fills the space restoring elastic recoil, reducing dynamic airway closure and gas trapping. The resulting decrease in residual volume returns the diaphragm to a favourable position for efficient ventilation (5).
Lung volume reduction (LVR) surgery
The National Emphysema Treatment Trial (NETT) continues to be the sentinel research underpinning current LVRS practise, defining patient populations for which the intervention confers benefit (6). Prior to this, case series and small randomised trials had suggested benefit (7,8) although patient numbers were modest. Wider concern was voiced about unacceptable mortality and morbidity associated with the procedure (9). The study was designed in response to these uncertainties (10).
The NETT trial randomly assigned 1,218 patients to either LVRS or best medical treatment using exercise capacity and mortality as primary outcome measures. Inclusion criterion included the presence of severe airway obstruction (FEV1 <45%), gas trapping (RV >150%) and hyperinflation (TLC >100%). All patients underwent pulmonary rehabilitation prior to trial entry.
The early results from the trial defined a patient population (n=140) at high risk of mortality, reaching 16% at 30 days P<0.001 (11).
- FEV1 <20% predicted and;
- DLCO <20% or homogeneous emphysema pattern.
The presence of these features continues to be an absolute contraindication to LVRS. Such patients randomised to the control group also had poorer prognosis; these clinical characteristics are therefore used within the current transplant guidelines for selection of appropriate patients.
Even after exclusion of high risk patients, NETT did not demonstrate a survival advantage between patients managed medically and surgically. Mortality results for “non-high risk” patients were dependent on post-hoc subgroup analysis stratified by the pattern of emphysema and patient’s exercise capacity. Maximal workload at cycle ergometry was used to define exercise capacity-low exercise capacity being less than 40 Watts for males and 25 Watts for females based on sex specific normal values.
The sub-groups were:
- Upper-lobe predominance, low base-line exercise capacity (n=290);
- Upper-lobe predominance, high base-line exercise capacity (n=419);
- Non-upper-lobe predominance, low base-line exercise capacity (n=149);
- Non-upper-lobe predominance, high base-line exercise capacity (n=220).
Of the four subgroups, only group 1 characteristics conferred a survival benefit during initial follow-up. Over an initial mean follow-up of 29.2 months, these patients undergoing LVRS had a significantly reduced risk of death (P<0.005). No benefit in survival was observed for those patients with non upper lobe emphysema regardless of their exercise capacity. The second primary endpoint of exercise capacity, did favour patients undergoing the procedure. A total of 52% of surgical patients improved exercise capacity defined as any improvement in cycle ergometry from baseline at 6 months compared to 20% of controls (P<0.001). This benefit extended to 24 months although the effect did diminished over time (31% in the surgical group compared to 10% controls had sustained improvement at 24 months).
Long term follow-up of the patient cohorts (12) confirmed the survival benefit to 5 years in the patients with upper-lobe emphysema and low exercise capacity (relative risk 0.67, P<0.003). Again, no survival advantage was demonstrated in the remainder of patients groups. The additional suggestion from this longer term data is the consideration of patients with upper lobe disease and high baseline exercise capacity as a palliative procedure. Significant improvements in quality of life as assessed by the St George’s Respiratory Questionnaire (SGRQ) were seen to 5 years.
The long term benefit in the selected patients above must be tempered with shorter term risk of surgery. The original study reported a 90 day mortality of 5.2% in non-high risk patients compared to 1.5% of those patients undergoing medical therapy. This higher mortality was not seen in the upper lobe predominant low exercise capacity patients for whom the procedure should be considered (2.9% 90 day mortality vs. 3.3% within the control group). Airleak occurred in 90% of patients (median duration 7 days) with 12% persistence at 30 days. Of patients undergoing LVRS, 28.1% remained hospitalised at 30 days. Airleak was universal in those patients not surviving 30 days although the low mortality rate at this time point (3.6%) meant a statistical association was not observed. Nevertheless, higher rates of adverse outcomes (pneumonia, ICU readmission, longer length of stay) were seen in patients with airleak (13). These peri-operative risks and the associated cost implications have contributed to the quest for less invasive bronchoscopic techniques for achieving LVR.
Surgical technique and considerations
The large numbers of patients enrolled in NETT provided an opportunity to compare techniques and outcomes (13,14). Individual centres had the option of using either video assisted thoracoscopic surgery (VATS), median sternotomy or internally randomising patients to either. Of the 552 patients randomised patients who underwent surgery, 69% underwent median sternotomy, with the remainder mostly undergoing a VATS procedure. Choice of operation did not affect mortality outcomes although VATS was associated with shorter ICU and hospital stay with consequential reduced cost (14).
The technique is usually a non-anatomical wedge resection aiming for LVR of 20-30% rather than an anatomical lobectomy (15). Staple lines are a common source of airleak. Prior small non-randomised and randomised studies had suggested that buttressing-reinforcement of stable lines with bovine pericardium or PTFE reduces length of stay (16) and airleak duration (17) with the practise widely applied amongst NETT patients. Patient factors rather than operative technique seemed to have a larger influence on outcome in the NETT cohort. There was no difference in proportion of patients with airleak or its duration when comparing procedure type or buttress material. Longer duration of airleak was associated with lower DLCO and FEV1, Caucasian ethnicity, use of inhaled steroids, pleural adhesions and upper lobe disease (13).
Non surgical methods for LVR
A number of bronchoscopic interventions have been proposed for non-surgical LVR (18-22). Facilitating LVR bronchoscopically may negate some of the risk associated with surgery, reduce inpatient stay for the procedure and potentially reduce the associated costs. Trial data comparable to the NETT study is not currently available for the majority of these interventions.
For the majority of these techniques, the NETT results have been extrapolated so that patients most likely to benefit can be targeted. Patients identified as ‘high risk’ by NETT criterion are usually excluded. Likewise most of the existing studies focus on heterogeneous emphysema distribution, usually in the upper lobes. Homogenous emphysema has been addressed with interventions such as airway bypass-endobronchial fenestrations with stenting and LVR coils (LVRCs). The aim of airway bypass is to reduce hyperinflation and gas trapping by creating extra-anatomical airways bypassing expiratory flow limitation utilising stents to maintain patency of the airway created. LVRCs aim to improve these parameters by improving small airway patency by applying traction forces across lung parenchyma thus reducing expiratory airway collapse.
Bronchoscopic interventions can be broadly divided into:
- Reversible airway interventions. These include endobronchial valves; LVRCs and transbronchial stents. These may potentially be retrieved if complications occur;
- Irreversible interventions inciting an inflammatory/fibrotic response or irreversibly plugging distal airways. These include bronchoscopic thermal vapour ablation (BTVA) and biological LVR (BioLVR).
Of these interventions the largest body of evidence is currently available for endobronchial valves, although as we will see collateral ventilation has limited its overall efficacy and translation to clinical practice. The current focus is on identifying and selecting patients without collateral ventilation for whom the technique may be of benefit. BTVA and LVRCs show promise although large scale randomised trials required to support their widespread use are currently pending or not available. The majority of these techniques rely on analysis of HRCT images via software packages to facilitate precise targeting of the most diseased lung parenchyma.
Endobronchial valves
Endobronchial valves allow unidirectional airflow. When sited in bronchi leading to hyper-expanded, emphysematous lung parenchyma, air is permitted to escape on expiration with no corresponding inspiratory flow. Lung distal to the stent, assuming no collateral ventilation, will collapse and become atelectatic. Resultant reduction in lung volume should have the same physiological effect to surgical LVR. At present two valve products are marketed (Zephr™ and IBV); despite differences in valve design the physiological principles for action are similar.
Results of the initial large randomised trial (VENT study) (23) were not as encouraging as the preliminary studies (24). A total of 321 patients were randomised to Zephr™ endobronchial valve placement or best medical care with a 2:1 ratio. A sham procedure was not undertaken in this study. Patients all had severe airflow obstruction and radiologically heterogeneous emphysema quantified on HRCT chest. Although the study showed statistically significant improvement in the primary outcomes at 6 months (FEV1 4.3% increase; 6MWT 9 meters improvement) the magnitude of these changes was deemed unlikely to be clinically meaningful (25). Pre-defined major complications were seen in 4.2% of patients undergoing valve therapy. Although not pre-defined as major complications, 7.9% and 5.6% of patients experienced an exacerbation of COPD requiring hospitalisation or haemoptysis respectively.
The European arm of the VENT trial (n=171) was commenced to support slow recruitment in the American study (26). Target recruitment was eventually achieved hence the European cohort being reported separately. Study design was similar to the American arm. When looking at the study population as a whole, a statistically significant improvement at 6 months was seen in only cycle ergometry (5 watts mean improvement compared to controls; P<0.05) and SGRQ. The change in SGRQ (5 points) was again below the threshold considered clinically meaningful. The reported focus on this second paper from the VENT group was the effect of collateral ventilation and complete lobar isolation. Subjects in the treatment arm underwent further evaluation with HRCT 6 months post procedure to assess degree of airway occlusion and volume reduction of the targeted lobe. Forty-four subjects in the treatment group of 111 had a complete fissure suggesting the absence of collateral ventilation. A complete fissure conferred reduction in lobar volume by 55% compared with 13% where the fissure was incomplete. Lobar isolation was seen in 48% of patients at 6 months (assessed by HRCT) indicating most patients continued to ventilate the targeted lobe despite the procedure. Combining these two variables (no collateral ventilation; successful technical isolation) yielded the most encouraging results. Improvements in FEV1, 6MWT and St George’s questionnaire were all clinically and statistically significant in this instance.
Ninane et al. tested IBV valves in a sham procedure controlled study (n=73) (27). Upper lobes were targeted although the study design was such that complete lobar occlusion was deliberately avoided to prevent lobar atelectasis which the study author hypothesised may cause adverse events. The primary outcome was proportion of patients responding to treatment by reaching a composite endpoint of change in SGRQ and lobar volume (defined as a 4-point increase in SRGQ, reduction in target lobe volume and 7.5% increase in lower lobe volume at HRCT assessment at 3 months). Although significantly more patients in the treatment group responded (8/33 vs. 0/35, P=0.002), the majority of patients did not respond to the treatment. The study design and avoidance of lobar atelectasis may account for the low proportion of responders.
The success of endobronchial valves is therefore highly dependent on lobar isolation and collateral ventilation which, as described above, occurs in a significant number of patients. Further techniques have been developed to assess CV (28). The Chartis system allows the targeted lobe to be occluded with an endobronchial balloon with measurement of expiratory airflow and pressure distal to the occlusion. Presence of flow distal to the balloon occlusion is suggestive of CV. This system can be used to determine which patients are more likely to respond to the insertion of endobronchial valves based on the measurement of CV (29). In this cohort of 96 patients undergoing endobronchial valve insertion 35% were assessed as having collateral ventilation present at bronchoscopy utilising Chartis. The system predicted response to insertion of endobronchial valves. Absence of CV conferred mean lobar volume reduction of 751 mLs compared to 98 mLs where CV was present (P<0.0001). These figures are clinically relevant as volume reduction in target lobe has been correlated with reduction in BODE index (body mass index, obstruction, dyspnoea and exercise tolerance) at 6 months (30).
The main limitation for using Chartis to assess collateral ventilation and predict which patients stand to benefit is the requirement for bronchoscopy. Patients with CV found at bronchoscopy precluding (or predicting poor response) to endobronchial valve placement would have undergone a procedure with limited potential for therapeutic benefit. At present this must be factored into the risk benefit analysis. Limiting Chartis assessment for CV to patients with complete fissures identified at radiology may improve the yield of bronchoscopic assessment identifying subject most likely to benefit from valve therapy. A trial addressing this question is currently recruiting (31). An alternative strategy might be to use an alternative irreversible CV independent technique in patients where CV is identified as described below.
Bronchoscopic thermal vapour ablation (BTVA)
This technique causes a thermal injury via heated water vapour to emphysematous lung to induce an inflammatory response. The resulting atelectasis and fibrosis reduces the volume within the targeted lung segment potentially conferring similar physiological effect to conventional LVRS. Unlike endobronchial valves, the technique is not dependent on collateral ventilation.
Snell et al. published a case series of 44 patients undergoing unilateral BTVA (32). Patients with severe airway obstruction (FEV1 15-45% predicted) were included if heterogeneous upper lobe emphysema was present as defined by lower lobe: upper lobe tissue to air ratios of >1.2 on baseline HRCT scan. This scan was used to plan treatment location and dose using predefined algorithms. In the above trial the 10 cal/gram dose of steam vapour was directed to the most diseased lung parenchyma. The targeted segments are intubated using a catheter directed through the bronchoscope working channel. A balloon is then fed over the guide catheter and inflated to protect the non-treated lung and airways prior to the predefined vapour dose being delivered (Figure 1). Follow-up to 6 months demonstrated encouraging results. Significant volume loss was seen in the targeted lobe (mean reduction 715 mL; P<0.001), FEV1 improved (141 mLs, P<0.001) as did 6MWT distance (46.5 metres, P<0.001). Symptomatic improvement was reported although these improvements must be interpreted with caution given the absence of a control group.
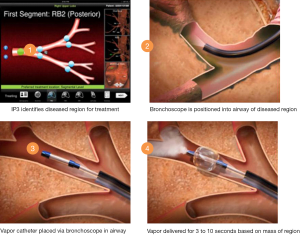
Given the mechanism of LVR-thermally induced lung injury and inflammation-it is unsurprising that respiratory complications were reported. A total of 25 of 29 adverse events were of a respiratory aetiology (43% of patients). COPD exacerbations and pneumonia were recorded in the 3 months following the procedure. A single death due to ‘end stage COPD’ was reported at 67 days. Follow-up analysis demonstrates that patients who experienced symptoms attributable to the localised inflammatory response derived greater benefit from the procedure in terms of volume reduction (33). A randomised phase III “Step-Up” trial is currently underway (34), recruiting 69 patients with heterogeneous bilateral upper lobe emphysema randomised 2:1 to either sequential bilateral upper lobe BTVA 3 months apart or best medical therapy. The treatment will clarify the role of this therapy and provide important safety data.
Lung volume reduction coils (LVRCs)
By applying traction forces to lung parenchyma, LVRCs aim to improve hyperinflation and gas trapping by reducing dynamic airway collapse (22). The mechanism of action is again independent of CV and could be applied to emphysema that is homogeneous or heterogeneous (in contrast to BTVA where heterogeneous disease is currently being targeted). The early published data shows promise with larger studies underway (35,36). The technique involves catheterising target lung segments with a guide wire to a distance 3.5 mm to the pleural edge (Figure 2). The coil sits within a loading sheath, straightening it prior to deployment. As the sheath and guide wire are withdrawn the LVRC reverts to its prior coiled shape applying traction to the surrounding lung parenchyma. Dynamic expiratory small airway collapse is reduced by application of radial traction thus improving gas trapping and hyperinflation. Up to ten LVRCs can be sited during a procedure initially unilaterally with further scope for a contra-lateral procedure at a later date if tolerated.
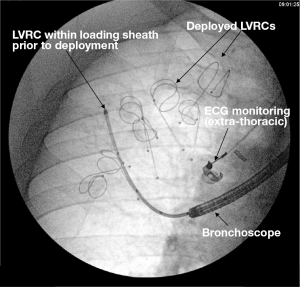
The most comprehensive evaluation of LVRCs was published as the RESET trial (35). Forty-seven patients were randomised to either LVRCs or usual care (1:1) with follow up to 90 days. Inclusion criterion included severe airflow obstruction (FEV1 <45%), emphysema on HRCT, TLC >100% and dyspnoea (MMRC score >2). Primary outcome was SGRQ with secondary outcomes including 6MWT, FEV1 and MMRC dyspnoea score. Although baseline characteristics were not matched, clinically and statistically meaningful improvements were seen in SGRQ (8.36 between group improvement P=0.04) and 6MWT distance (63.55 metre between group improvement, P<0.001). No improvement in TLC was seen at 90 days. Further studies are required and are currently recruiting to further evaluate this technique in larger cohorts of patients (35).
Biologic lung volume reduction (Bio-LVR)
The principle of bio-LVR is similar to that of bronchoscopic thermal ablation. A fibrinogen based biopharmaceutical suspension containing thrombin polymerises when instilled into targeted airways (20). The resulting biodegradable matrix induces a localised inflammatory response inducing fibrosis and collapse of the targeted segment. Nonrandomised phase II studies evaluating optimal dose and safety demonstrated significantly improved FEV1, RV/TLC ratio and RV in 22 patients undergoing higher dose (37). The treatment was associated with transient fevers, leukocytosis and COPD exacerbations. Despite promise, phase III trials were not further pursued, presumably due to the development of the alternative preparation Aeriseal® by the study sponsor.
In contrast to bioLVR, the Aeriseal® preparation aims to induce LVR acting at bronchiolar and alveolar levels by sealing airways inducing absorption atelectasis thus leading to reduction in lung volume. The proposed mechanism may also obscure collateral ventilation pathways. Non-randomised case series have examined the safety of this intervention (38). Magnussen et al.’s later case series is the most comprehensive evaluation of the intervention (39). Fifty-four patients with Global Initiative for Obstructive Lung Disease (GOLD) stage III or IV COPD, gas trapping RV >135% (mean 242%) and hyperinflation were evaluated with HRCT to assess for upper lobe emphysema. All included patient were treated with Aeriseal at 2-4 subsegemental sites and followed to 12 weeks. The authors further divided the cohort into patients for whom data with regard to fissure integrity was available. In this subset of 28 patients TLC reduced by 214 and 261 mLs in patients with and without complete fissures respectively. There was no significant difference between the magnitude of change when assessing for the presence of radiologically intact fissures suggesting the treatment is independent of CV. Six-minute walk distance improved by a mean of 31.9 metres with 31% of patients achieving a clinically meaningful improvement of 54 metres. Despite promise the phase III trial was terminated by the study sponsor in November 2013 prior to publication (40). At present the only registered trial recruiting is a phase II study evaluating the role of autologous blood as a biological irritant to induce LVR (41). Given the absence of phase III trials actively recruiting, it is unlikely that biological methods of LVR will implemented into routine clinical practise in the near future.
Endobronchial and extra-pulmonary bypass procedures
Airway bypass procedures have been proposed to reduce gas trapping by directly relieving trapped air in emphysematous lung by creating extra-anatomical airways. Bronchoscopic fenestrations between large airways and diseased lung parenchyma are created to improve expiratory flow. Drug eluting stents are then sited in an attempt to maintain ongoing patency of the novel tracts. The procedure was proposed for those patients with homogenous (diffuse) emphysema. Unfortunately the large (n=315), randomised, sham procedure controlled study evaluating the technique showed disappointing results (42). Improved FVC immediately post procedure was not sustained past 1 month. There was no difference in MMRC dyspnoea scale. Adverse events occurred at higher frequency in the treatment group although serious adverse events were rare. The authors hypothesised that lack of sustained response likely related to occlusion of the stent with mucus or granulation tissue. At present there is no role for the technique-whether changes to stent design might improve long term efficacy remains unevaluated.
An alternative extra-anatomical approach has been suggested and is in early developmental stages (43,44). Expiratory flow rates may be augmented by surgically creating a fistula between the diseased hyper-inflated lung parenchyma and the chest wall thus reducing hyperinflation. The larger calibre bypass airway created is likely to be less prone to occlusion than transbronchial airway stents. The initial case series (six patients) utilised an improvised endotracheal tube to maintain airway patency. Custom designed pneumonectomy catheters-the ‘portaero pneumostoma’ have subsequently been developed and are under evaluation (45). The risk benefit profile for this method of LVR will require careful evaluation (Figure 3).
Lung transplantation
Indications for lung transplantation in COPD
Despite significant symptoms and functional limitation patients with advanced COPD have survival which is variable due, generally, to slow chronic disease progression over years. Median survival of patients with GOLD stage III and IV disease is 6 years (46). After transplantation, patients with COPD have median survival of 5.4 years with 30% of transplanted patients surviving to 10 years (47). Given that goals of transplantation are improvement of symptoms and survival, patient selection and identification of subgroups of patients with poor prognosis is critical. The presence of severe airway obstruction alone is insufficient to predict who might benefit. Whether lung transplantation should be offered to palliate symptoms without improvement in survival benefit is contentious, especially given limited availability of donor organs (48). In general terms, lung transplantation is indicated where predicted survival is less than 2 years in patients with NYHA III or IV symptoms and associated poor quality of life. The presence of absolute or relative contraindications must be considered and factored into clinical decision making when proceeding to transplant. (Tables 1,2) (48).

Full table
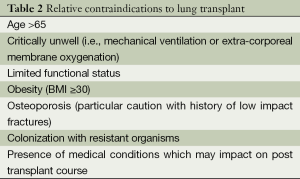
Full table
Patients should ideally be referred to a transplant centre before they are established in the “transplant window”-the time period for which the patient is likely to confer benefit from transplantation prior to becoming too frail to undertake the peri operative rigours and recovery after transplantation. This allows adequate time for assessment, consideration of alternative options (i.e., LVRS as discussed above) and addressing reversible relative contraindications or issues that may impact on the transplant process. Factors which should prompt referral to a transplant unit in patients considered appropriate are outlined in Table 3.
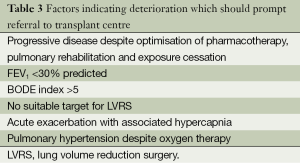
Full table
Acute COPD exacerbations with associated hypercapnia (PCO2 >50 mmHg) confer a poorer prognosis with associated 2-year median survival of 49% (49). This study was performed prior to NIV becoming routine for exacerbations associated with hypercapnia. A total of 89% of the study cohort survived the index admission which suggests that such exacerbations may be a marker for progressive disease and death.
Scoring systems may also have a role in identifying patients with poor prognosis (50). The BODE score further uses body mass index (B), degree of obstruction (O), dyspnoea (D)-MMRC dyspnoea scale, and exercise capacity (E)-6 minute walk test (6MWT) to stratify which patients have poorer prognosis. Scores of 7-10 confer median survival of 3 years indicating patients are symptomatic, functionally limited and are likely to have a survival benefit from transplantation. The NETT trial also identified a subgroup of patients with poor prognosis. Subjects who did not undergo LVRS (control group) with low FEV1 (<20%), and either low DLCO (<20%) or homogenous emphysema survived for a median of 3 years although this was significantly better than similar patients undergoing LVRS. Patients with refractory pulmonary hypertension despite oxygen therapy should also be considered given high waiting list mortality (51).
In appropriately selected patients, lung transplant is associated with significant improvements in quality of life and exercise capacity (52,53). Despite COPD being the leading indication for lung transplantation accounting for 33.5% of procedures worldwide, it remains a highly limited resource. The 12,602 procedures have been performed for this indication worldwide between 1995 and 2012. In the United States the lung allocation score (LAS) was introduced to objective prioritise patients on the transplant waiting lists at highest risk of mortality (54). Whilst this intervention has improved waiting time and mortality for patients with idiopathic pulmonary fibrosis, conversely COPD patients can expect to wait longer for lung allocation (55). The main barriers limiting transplantation to a minority of patients are donor organ availability and cost. Increasing the numbers of organs available for transplant can be achieved either by:
- Increasing the percentage of eligible donors identified or consenting to transplant. Large variation in organ donation rates worldwide reflect legal, cultural and organisation differences and has been comprehensively reviewed elsewhere (56);
- Changing retrieval techniques and practises. The emerging practise of donation after circulatory death (DCD), in addition to the more conventional brainstem death donors;
- Improving utilisation rates of organs offered for transplantation using novel technologies such as ex vivo lung perfusion (EVLP).
Donation after circulatory death (DCD)
DCD is not a new concept, reintroduced clinically in 1995 (57), but not widely practised due to concerns about prolonged warm ischaemic time and inferior organ assessment opportunity. Donation after brain stem death (DBD) has been the traditional source of donor lungs. Over the last decade, DCD has emerged as a significant pool of donor organs enabling an increase in transplant volume. Since the 2006 introduction of lung DCD programmes in Australia, 12.4% of organs have been acquired from DCD (58). By 2010 this represented an extra 28% of donors being utilised. The Maastrict classification established in 1995 describes the different circumstances whereby DCD organ donors may be procured (59). Briefly, Maastrict categories I and II refer to uncontrolled deaths in patients deceased on arrival at hospital or with unsuccessful resuscitations attempts respectively. Category III-death after controlled withdrawal of supportive treatment (usually in an intensive care unit) describes the majority of DCDs in Australia, USA and Europe (excluding France and Spain where category II donors are more common) (60). Categories IV and V refer to circulatory collapse after brainstem death and inpatient cardiac arrests respectively-these are not common modes of organ procurement.
Clinical outcomes of patients receiving DCD lungs are comparable to that of conventional lung donors (58,61,62). The Australian DCD collaborative is the largest reported series of exclusively Maastrict III donors (58). Short and long term DCD outcomes are similar to that of DBD patients over the same time period. Among 72 patients receiving DCD lungs, 1 and 5 year survival was reported at 97% and 90% respectively (90% and 60% for 503 patients undergoing DBD during the same time period). Incidence of primary graft dysfunction (PGD) and bronchiolitis obliterans syndrome was similar between groups. This supports the notion that group III DCD donors which otherwise meet conventional acceptance criterion (Table 4) should not be considered ‘marginal’. This is in contrast to practise in other centres where EVLP has been routinely employed for all DCD lungs (63).

Full table
Ex vivo lung perfusion (EVLP)
Lung transplantation is dependent on the availability of organs from suitable donors. Respiratory complications in potential lung donors contribute to a low proportion of organs proceeding to transplantation. Common donor mechanisms of death-chest trauma, aspiration, ventilator associated pneumonia, barotrauma and systemic inflammatory response syndrome all impact on organ utility. Transplant physicians exercise caution when assessing potential donor lungs to minimise the risk of morbidity and mortality from PGD-a condition associated with inferior short and long term outcomes (64). It is seen more frequently in patients where there is deviation from traditional donor acceptance criterion (Table 4) (65). These parameters will minimise the risk of PGD but lead to a low proportion of potential donors converting to transplant. Of organs offered for transplant a low proportion—15% to 20%—are utilised (66). Strategies to safely increase the number of “marginal” donors-those organs with clinical features/parameters deviating from traditional acceptance-will have an impact on numbers of patients able to undergo transplantation. Reported results from some larger transplant centres suggest those traditional acceptance criterions are overly stringent (67) with transplantation being safely undertaken where the donor does not fully adhere to this criteria. Recognition that these criteria are not absolute may be contributing to recovery of a higher proportion of organs (68). EVLP is a further tool that has potential to further improve this trend.
EVLP is used in the assessment and reconditioning of donor lungs. The technique was first introduced by Steen et al. in 2001 for graft assessment after Maastrict II DCD (69). The Toronto group recognised the potential of the technique for addressing donor respiratory complications. Refinement to the process means that lungs previously discarded can be reconditioned, re-assessed and if suitable transplanted (70). Potential indications for the use of EVLP although not standardised reflect deviation from traditional acceptance criterion (63,71):
- PaO2/ FiO2 <300 mmHg with PEEP 5 cm H2O;
- Infiltrates on CXR (pulmonary oedema/pneumonic consolidation);
- Poor lung compliance or PEEP dependent donor lungs;
- Questionable aspiration history;
- Logistical difficulties resulting in anticipated prolonged cold ischaemic time.
As outlined above, procurement of DCD donors has been used as an indication for EVLP (63) although other centres have demonstrates satisfactory DCD outcomes without this additional assessment (58). Controversy exists with regard to EVLP in where it should be employed. As mentioned above, a proportion of marginal donors can be utilised without EVLP assessment without compromising outcomes (67); given this more work is required to redefine the boundaries of donor conventional donor acceptability. Such studies may define where marginal lungs could be utilised without EVLP-without this information there is a risk that the technique could become standard of care prior to these limits being clarified.
The EVLP circuit consists of a sterile chamber housing the donor lungs, centrifugal pump circulating the perfusate, leucocyte filter and membrane de-oxygenator (Figure 4). Two differing protocols are currently used and referred to as Lund protocol (72) and Toronto protocol (64), although the general principles are common to the two methods. The perfusate provides above normal oncotic pressure and inhibits endothelial leucocyte interaction, generation of reactive oxygen species and thrombogenesis. Gradual warming of the solution occurs to 37 °C allowing restoration of cellular metabolic pathways permitting return to physiological conditions at normothermia. Antibiotics can be administered and interstitial oedema improved via hyperosmolar perfusate mediated fluid shifts. Lungs are connected at an initial perfusate temperature of 15 °C; at a temperature of 32 °C gentle ventilation is commenced with recruitment manoeuvres enabling re-expansion of lobar or segmental collapse. Bronchoscopy may also be performed to assess for and remove secretions from the tracheo-bronchial tree.
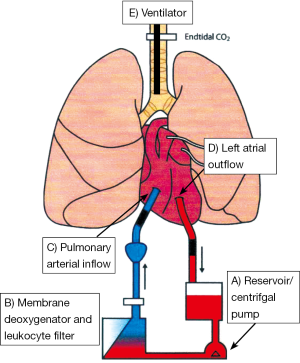
Initial data suggests that outcomes with EVLP are similar to conventional lung transplants (63,71,73,74). The HELP study prospectively assessed the role of EVLP in a non-randomised clinic trial (63). A total of 306 donor offers were assessed; 111 donors proceeded directly to transplant whilst 23 underwent EVLP management having met pre-defined high risk criterion. Of these EVLP conditioned donor lungs 20 were successfully transplanted (3 EVLP assessments were deemed unsatisfactory for transplant). No significant differences in PGD or mortality were seen to 30 days compared with control subjects undergoing standard transplantation procedure. The same group report later reported EVLP conditioned lungs accounting for 20% of their transplant activity-significant given these organs would otherwise not be utilised (71). Larger multicentre trials are currently underway aiming to confirm these preliminary findings—that EVLP can be safely used to increase donor number (75).
Conclusions
Despite the high prevalence of advanced COPD, current therapeutic options in medically optimised patients are available to a minority. For LVRS, the NETT trial showed that patient selection is critical to outcome and limits the availability to those patients with heterogeneous upper lobe disease. The procedure comes with a risk of morbidity and mortality which has led to the development of less invasive methods of LVR. With time, these may improve accessibility for patients. At present the evidence is insufficient to firmly recommend bronchoscopic LVR methods. Endobronchial valves, the most comprehensively evaluated technique, require lobar isolation and CV to be absent. Work is currently underway to further develop patient selection pathways to prospectively predict who may benefit. Non CV dependent techniques (BTVA and LVRCs) are promising, but require larger randomised trials to confirm efficacy and their safety. In patients for whom LVR is not an option due to absence of an LVR target or contraindications, lung transplantation may be considered. Its widespread application is limited by cost, rigorous selection criterion and organ availability. Work is underway to improve the accessibility of this limited resource. EVLP is an emerging technique which may assist with this by increasing the proportion of potential donors utilised with early data suggesting such transplants comparable to conventional procedures. Further work is required to define indications for EVLP and conversely circumstances where conventional organ acceptance criterion can be confidently extended.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- World Health Organisation. The world health report 2000-Health systems: improving performance. Available online: http://www.who.int/whr/2000/en/
- Global initiative for Chronic obstructive lung disease. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease. Available online: http://www.goldcopd.org
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412. [PubMed]
- Loring SH, Leith DE, Connolly MJ, et al. Model of functional restriction in chronic obstructive pulmonary disease, transplantation, and lung reduction surgery. Am J Respir Crit Care Med 1999;160:821-8. [PubMed]
- Gorman RB, McKenzie DK, Butler JE, et al. Diaphragm length and neural drive after lung volume reduction surgery. Am J Respir Crit Care Med 2005;172:1259-66. [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [PubMed]
- Criner GJ, Cordova FC, Furukawa S, et al. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:2018-27. [PubMed]
- Cooper JD, PattersonGA, Sundarsen RS, et al. Results of 150 consecutive lung volume reduction procedures in patients with severe emphysema. J Thorac cardiovasc Surg 1996;112:1319-29; discussion 1329-30. [PubMed]
- Health care financing administration. Report to congress: lung volume reduction surgery and medicare coverage policy: implications of recently published evidence. Baltimore, MD, USA, 1998.
- Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. The National Emphysema Treatment Trial Research Group. Chest 1999;116:1750-61. [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [PubMed]
- Naunheim KS, Wood DE, Mohsenifar Z, et al. Long term follow-up of patients receiving lung volume reduction surgery versus medical therapy for severe emphysema. Ann Thorac Surg 2006;82:431-43. [PubMed]
- Decamp MM, Blackstone EH, Nauheim KS, et al. Patient and surgical factors influencing airleak after lung volume reduction surgery: lessons learned from the initial national emphysema treatment trial. Ann Thorac Surg 2006;82:197-207. [PubMed]
- McKenna RJ Jr, Benditt JO, DeCamp M, et al. Safety and efficacy of median sternotomy versus video-assisted thoracic surgery for lung volume reduction surgery. J Thorac Cardiovasc Surg 2004;127:1350-60. [PubMed]
- Russi EW, Stammberger U, Weder W. Lung volume reduction surgery for emphysema. Eur Respir J 1997;10:208-18. [PubMed]
- Hazelrigg SR, Boley TM. Effect of bovine pericardium strips on airleak after stapled pulmonary resection. Ann Thorac Surg 1997;63:1573-5. [PubMed]
- Stammberger U, Klepetko W, Stamatis G, et al. Butressing the staple line in lung volume reduction surgery: a randomised three-centre study. Ann Thorac Surg 2000;70:1820-5. [PubMed]
- Toma TP, Hopkinson NS, Hillier J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003;361:931-3. [PubMed]
- Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 2009;88:1993-8. [PubMed]
- Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med 2003;167:771-8. [PubMed]
- Cardoso PF, Snell GI, Hopkins P, et al. Clinical application of airway bypass with paclitaxel-eluting stents: early results. J Thorac Cardiovasc Surg 2007;134:974-81. [PubMed]
- Herth FJ, Eberhard R, Gompelmann D, et al. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis 2010;4:225-31. [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [PubMed]
- Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest 2006;129:518-26. [PubMed]
- Wise RA, Brown CD. Minimal clinically important differences in six minute walk test and the incremental shuffle walking test. COPD 2005;2:125-9. [PubMed]
- Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. [PubMed]
- Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur respir J 2012;39:1319-25. [PubMed]
- Aljuri N, Freitag L. Validation and pilot clinical study of a new bronchoscopic method to measure collateral ventilation before endobronchial lung volume reduction. J Appl Physiol (1985) 2009;106:774-83. [PubMed]
- Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinincal outcomes of using Chartis to plan endobronchial valve treatments. Eur Respir J 2013;41:302-8. [PubMed]
- Valipour A, Herth FJ, Burghuber OC, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J 2014;43:387-96. [PubMed]
- Endoscopic Lung Volume Reduction After Catheter-based CV Measurement in Patients With Heterogeneous Emphysema and Complete Interlobar Fissures. Available online: http://www.clinicaltrials.gov. NCT01902732.
- Snell G, Herth FJ, Hopkins P, et al. Bronchoscopic thermal vapour ablation therapy in the management of heterogeneous emphysema. Eur Respir J 2012;39:1326-33. [PubMed]
- Gompelmann D, Eberhardt R, Ernst A, et al. The localized inflammatory response to bronchoscopic thermal vapor ablation. Respiration 2013;86:324-31. [PubMed]
- Sequential Segmental Treatment of Emphysema With Upper Lobe Predominance (STEP-UP) Study. Available online: http://www.clinicaltrials.gov. NCT01719263.
- Shah PL, Zoumat Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013;1:233-40. [PubMed]
- Lung Volume Reduction Coil Treatment in Patients With Emphysema (RENEW) Study. Available online: http:// www.clinicaltrials.gov. NCT01608490.
- Criner GJ, Pinto-Plata V, Strange C, et al. Biologic lung volume reduction in advanced upper lobe emphysema: phase 2 results. Am J Respir Crit Care Med 2009;179:791-8. [PubMed]
- Herth FJ, Eberhardt R, Ingenito EP, et al. Assessment of a novel lung sealant for performing endoscopic volume reduction therapy in patients with advanced emphysema. Expert Rev Med Devices 2011;8:307-12. [PubMed]
- Magnussen H, Kramer MR, Kirsten AM, et al. Effect of fissure integrity on lung volume reduction using a polymer sealant in advanced emphysema. Thorax 2012;67:302-8. [PubMed]
- Study of the AeriSeal System for HyPerInflation Reduction in Emphysema (ASPIRE). Available online: http://www.clinicaltrials.gov. NCT01449292.
- Low Cost Biological Lung Volume Reduction Therapy for Advanced Emphysema (BLVR). Available online: http://clinicaltrials.gov. NCT02107209.
- Shah PL, Slebos DJ, Cardoso PF, et al. Bronchoscopic lung-volume reduction with exhale airway stents for emphysema (EASE trial): randomised, sham controlled multicentre trial. Lancet 2011;378:997-1005. [PubMed]
- Moore JM, Cetti E, Haj-Yahia S, et al. Unilateral extrapulmonary airway bypass in advanced emphysema. Ann Thorac Surg 2010;89:899-906, 906.e1-2.
- Saad Junior R, Dorgan Neto V, Botter M, et al. Therapeutic application of collateral ventilation with pulmonary drainage in the treatment of diffuse emphysema: report of the first three cases. J Bras Pneumol 2009;35:14-9. [PubMed]
- A Non-randomized Study to Evaluate the Safety and Performance of the Portaero Pneumostoma System in Patients with Severe Emphysema and Hyperinflation of the Lung. ACTRN12610000190000. Available online: http://www.anzctr.org.au (Recruitment Status: Recruiting).
- Shavelle RM, Paculdo DR, Kush SJ, et al. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III Follow-up Study. Int J Chron Obstruct Pulmon Dis 2009;4:137-48. [PubMed]
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [PubMed]
- Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbations of severe chronic obstructive lung disease: the SUPPORT investigators (Study to Understand Prognosis and Preferences for Outcomes and Risks of Treatment). Am J Respir Crit Care Med 1996;154:959-67. [PubMed]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [PubMed]
- Egan TM, Bennett LE, Garrity ER, et al. Predictors of death on the UNOS lung transplant waiting list: results of a multivariate analysis. J Heart Lung Transplant 2001;20:242. [PubMed]
- Lanuza DM, Lefaiver C, Mc Cabe M, et al. Prospective study of functional status and quality of life before and after lung transplantation. Chest 2000;118:115-22. [PubMed]
- Cassivi SD, Meyers BF, Battafarano RJ, et al. Thirteen-year experience in lung transplantation for emphysema. Ann Thorac Surg 2002;74:1663-9; discussion 1669-70.
- Colvin-Adams M, Valapour M, Hertz M, et al. Lung and heart allocation in the United States. Am J Transplant 2012;12:3213-34. [PubMed]
- Nathan SD, Shlobin OA, Ahmad S, et al. Comparison of wait times and mortality for idiopathic pulmonary fibrosis patients listed for single or bilateral lung transplantation. J Heart Lung Transplant 2010;29:1165-71. [PubMed]
- Rudge C, Matesanz R, Delmonico FL, et al. International practices of organ donation. Br J Anaesth 2012;108:i48-55. [PubMed]
- Love RB, Stringham JC, Chomiak PN, et al. Successful lung transplant using a non-heart-beating donor. J Heart Lung Transplant 1995;14:88.
- Levvey BJ, Harkess M, Hopkins P, et al. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative. Am J Transplant 2012;12:2406-13. [PubMed]
- Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc 1995;27:2893-4. [PubMed]
- Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, et al. Current situation of donation after circulatory death in European countries. Transpl Int 2011;24:676-86. [PubMed]
- Mason DP, Brown CR, Murthy SC, et al. Growing single-center experience with lung transplantation using donation after cardiac death. Ann Thorac Surg 2012;94:406-11; discussion 411-2. [PubMed]
- De Vleeschauwer SI, Wauters S, Dupont LJ, et al. Medium-term outcome after lung transplantation is comparable between brain-dead and cardiac-dead donors. J Heart Lung Transplant 2011;30:975-81. [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [PubMed]
- Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short- and long-term outcomes. Semin Respir Crit Care Med 2010;31:161-71. [PubMed]
- Botha P. Extended donor criteria in lung transplantation. Curr Opin Organ Transplant 2009;14:206-10. [PubMed]
- Punch JD, Hayes DH, LaPorte FB, et al. Organ donation and utilization in the United States, 1996-2005. Am J Transplant 2007;7:1327-38. [PubMed]
- Snell GI, Griffiths A, Levvey BJ, et al. Availability of lungs for transplantation: exploring the real potential of the donor pool. J Heart Lung Transplant 2008;27:662-7. [PubMed]
- Valapour M, Skeans MA, Heubner BM, et al. OPTN/SRTR 2012 Annual Data Report: lung. Am J Transplant 2014;14:139-65. [PubMed]
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825-9. [PubMed]
- Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319-25. [PubMed]
- Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6. [PubMed]
- Steen S, Liao Q, Wierup PN, et al. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg 2003;76:244-52; discussion 252. [PubMed]
- Zych B, Popov AF, Stavri G, et al. Early outcomes of bilateral sequential single lung transplantation after ex-vivo lung evaluation and reconditioning. J Heart Lung Transplant 2012;31:274-81. [PubMed]
- Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [PubMed]
- Novel Lung Trial: Normothermic Ex Vivo Lung Perfusion (Evlp) As An Assessment Of Extended/Marginal Donor Lungs. Available online: http://www.clinicaltrials.gov. NCT01365429.


