JTD special edition ‘Hot Topics in COPD’—The microbiome in COPD
Introduction
Throughout history many inventions have radically altered the way we are able to perceive the world—the telescope, the microscope, lenses for those with refractive errors and cochlear implants for those with deafness. A similar revolution has recently occurred in the way we are able to observe the microbial world. The genomic methods and biostatical approaches which can now be employed to comprehensively determine the structure of microbial communities have transformed the study of microbial ecology and will have a similar impact on our understanding of human health. What should be self-evident—that we have evolved not in a sterile world, but in one teeming with microbes (our cells are outnumbered 10:1 by our gut bacteria alone) where each species has their own evolutionary agenda, but is also open to collaboration—has been brought into clear focus. The idea that infectious disease is a two-sided battle to the death has been exposed as simplistic, with symbiotic relationships between host and microbe being at least as prevalent and important to human health in some organs (especially the gut, but possibly also the lung). The implications for diseases like COPD where microbial colonisation and infection is central to pathogenesis are obvious and are the subject of this perspective.
What is the microbiome and how can we ‘see’ it?
The microbiome can be defined as the collective sum of microorganisms (and their genomes) inhabiting a given ecosystem. In the case of the human microbiome that ecosystem is us. Traditional microbiological methods have been blind to the majority of the microbiome for the simple reason that most microorganisms have not been readily grown in the laboratory and thereby become amenable to manipulation and interrogation at the human-scale. Things began to change in the 1980s when it was recognised that microorganisms could be catalogued by their gene sequences, primarily the highly conserved 16S rRNA marker gene, without the need to grow them (1). This was the birth of culture-independent molecular microbiology. First, bulk nucleic acids are extracted from a biological sample and the target gene is PCR-amplified from the extracted DNA.16S rRNA is so conserved that it is possible to target all cellular lifeforms using “universal” PCR primers, which allows essentially all microorganisms in a given sample to be amplified in one PCR reaction. The mixture of 16S rRNA amplicons were then historically separated by cloning into E. coli, but now are sequenced in parallel using cloneless next generation sequencing technologies such as 454 pyrosequencing or Illumina sequencing. The 16S rRNA amplicon sequences are then quality trimmed, compared to each other and typically clustered into operational taxonomic units (OTUs) and finally identified against a reference database. The number of reads assigned to each OTU for a given sample provides relative abundance information to create a community profile, an example of which is provided in Figure 1. Through such cataloguing surveys, it quickly became apparent that most microbial (evolutionary) diversity, roughly 85%, was not captured by our collection of domesticated microbial isolates (2). Moreover, in many instances, microorganisms isolated from a given habitat were, at best, bit players in ecosystem function (3). Combining 16S rRNA microbial profiling with ‘metadata’ such as disease state, physiology, etc., has proven to be a powerful way of identifying statistically significant correlations between the microbiome and disease that can be used for example as diagnostic biomarkers (4). Today, gene-based surveys are still heavily used in microbiome research, but they have also evolved into genome-based (metagenomic) surveys courtesy of greatly improved sequencing and computational technologies (5). Taking a census of the total genetic inventory of a given ecosystem not only tells us who is there, but also what they are potentially capable of doing. Complementary molecular methods such as metatranscriptomics and metaproteomics, which identify mRNA transcripts and proteins respectively, can tell us which genes and pathways are being expressed under a given set of conditions (5). Consequently our understanding of the microbiome in humans has exploded in recent years (6).
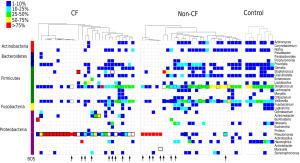
The ‘normal pulmonary microbiota’—does it exist?
Even today most medical students are taught that the lung is sterile. This idea was founded on data obtained in an era when non-selective genomic techniques were unavailable, so that microbial identification relied on basic general culture systems that were blind to 99% of bacterial species. Community profiling and metagenomic approaches now facilitate comprehensive characterization of the human microbiota and analysis of its role in health and disease. Given the extraordinary ability of microbes to adapt to even very hostile environments it seems unlikely that complete sterility of the lower respiratory tract that is continually exposed to the environment could be maintained, at a reasonable energy cost, given the enormous microbial load delivered with each breath. Although a number of studies purporting to confirm the presence of a healthy pulmonary microbiome have been published (7-10), all are in some way (as openly acknowledged by the authors) methodologically deficient either due to the acquisition of lower respiratory tract samples bronchoscopically (7-9), where oropharyngeal contamination is impossible to exclude, and/or due to the acquisition of lower respiratory tract material from potential organ donors (10) or lung resections (10). Organ donors will have invariably experienced gastric aspiration and/or ventilator associated bacterial colonization/infection (11), and lung resections from healthy subjects are difficult to obtain. A further difficulty is that the normal pulmonary microbiota, should it exist, will be many log lower than the surrounding human biomass, complicating DNA amplification and sequencing. Despite these deficiencies, the emerging model is that the human respiratory tract is not neatly compartmentalized into upper and lower tracts and hence the question of lung sterility is not a binary one. Intermittent colonisation of the lower respiratory tract occurs, even in healthy individuals, with more persistent or permanent colonisation being common in certain scenarios. Dickson et al., recently borrowed the ‘adapted island model’ from ecology to describe this pulmonary biogeography (12). While beyond the scope of this review, their work provides a convincing and useful framework for understanding lung ecology, and provides an intriguing perspective from which to view pneumonia. In their proposed model pneumonia does not occur as the result of a large inoculum of a pathogenic species overwhelming host defences, but as a small but snowballing disruption in the complex adaptive lung microbial ecosystem (12). A COPD exacerbation could be viewed in the same way. In summary, while these new models provide a more realistic framework for understanding the interactions between the human lung and the respiratory tract microbiota, the fundamental question remains whether this interaction is so intimate as to be classified as mutualistic or symbiotic.
Confirmation of such a relationship would imply benefit for both the microbiota and the host and would carry profound implications for our understanding of lung health. At the most basic level, we would need to reconsider what ‘self’ means in the context of lung immunology. In the best studied human system—the gut—it is clear that a healthy microbiota is critical to the development of both local and systemic immune responses and the maintenance of epithelial integrity. Perturbations in this symbiotic relationship (‘dysbiosis’) have been implicated in the pathogenesis of inflammatory bowel disease and the metabolic syndrome (13,14). Much of the literature on COPD microbiology has been written with the idea that the now colonised/infected lung was previously sterile. It is intriguing to think that a normal pulmonary microbiota may exist and that the act of its displacement by other organisms may in itself be detrimental to host health. In the lung, dysbiosis could predispose the host to excessive immune activation and/or loss of epithelial integrity—key features of multiple lung diseases including asthma, COPD and idiopathic pulmonary fibrosis. It could be that cigarette smoke induced dysbiosis, or disruption of lung biology by cigarette smoke, in conjunction with host genetic factors, may be important in COPD pathoegenesis, and, as a corollary, that restoring the microbiome could improve host health, opening the door to more subtle and more nuanced, but potentially highly effective, therapies.
What’s known about the microbiome in COPD?
Only a handful of studies have been published exploring the role of the lung microbiome in COPD and therefore our understanding this interaction remains in its infancy. Due to the heterogeneity of COPD features, particularly as they relate to disease severity, various studies have sought to evaluate the lung microbiome in stable or exacerbating disease states, in healthy patients vs. those with COPD, or in COPD compared to unrelated lung diseases such as cystic fibrosis (CF) or asthma. They have assessed the microbiome from differing parts of the airways and via different sampling techniques. As a result of these issues, the data that has resulted are not easily compared with each other and lack consistency.
Nevertheless these studies have produced valuable associative data on the changes in the microbiome in COPD. The major phyla that are associated with the normal lung microbiome appear to be Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria, and most studies agree that this is regardless of the presence of COPD, its status or severity (8-10,15,16). These studies have demonstrated no apparent difference in the total bacterial load nor in diversity. However, principle component analyses have revealed that the microbiome of COPD patients clusters separately (and therefore differs) from that of healthy controls. This is partly driven by increases in members of the phylum Firmicutes in patients with stable COPD (8,10,15,16). In severe COPD, however, the microbiome is unlike that of mild disease. In two notable studies using lung explants (2,3) and one using sputum (17) from severely affected COPD lungs compared to healthy controls, a shift was observed to a decrease in lung microbiome diversity driven partly by increases in Proteobacteria. This correlated with a shift towards the dominance of particular bacterial genera, especially Pseudomonas although one must consider that this effect could be influenced by bacterial induced exacerbations. Three studies have demonstrated a decrease in diversity during exacerbations and with antibiotic treatment and a further decrease in diversity with increasing severity and persistence of these disease flares (17-19). As a majority of all COPD exacerbations are associated with respiratory infections (20) this finding is not surprising. Nevertheless, no study has definitively shown that a reduction in COPD microbiome diversity in the lung has caused the outgrowth of a particular bacterial genera or an increase in bacterial load. This is difficult to assess in humans but may be investigated using animal models that are representative of the human condition (21-24).
One important issue with investigations thus far is sample collection. Cabrera-Rubio et al., assessed the microbiome from bronchial aspirates, sputum, bronchial lavage (BAL) and bronchial mucosal brushings. They found that the microbial diversity was lower and the load was higher in samples collected from the upper (aspirate and sputum) compared to the lower respiratory tract (BAL and mucosa). Furthermore, Erb-Downward et al., found that the microbial community in tissue from lung explants differed depending on the location from which the sample was taken, even within same patient (8). This indicates that the COPD lung microbiome is not homogeneous.
Recently it has become clear that inhaled corticosteroid use in COPD is associated with an increased risk of pneumonia (25,26). At present this observation, whilst very robust, remains empiric with little understanding of the mechanisms that lead to pneumonia, who may be at highest risk, and how that risk may be mitigated in the face of continued drug exposure. It is likely that answers to these highly relevant and clinically important questions will come through the deeper understanding of bacterial ecology in the COPD lung which is now obtainable with ecogenomic approaches (19).
Host-pathogen interactions in lung disease
Whilst exposure to environmental irritants, particularly cigarette smoke, is obviously central to COPD pathogenesis, airway infection also plays a role both in exacerbations and in disease progression even during the ‘stable’ phase of the illness (27). However, not all bacteria are created equal in this regard. For the best-studied pathogen, Haemophilus influenzae, it is clear that some strains induce more inflammation (particularly IL-8 induced neutrophilic inflammation) than others (16). However, given that most of the literature in this field was developed using traditional microbial techniques, the roles and/or impact of bacterial ecosystem was not incorporated into these studies.
When a host encounters a potential threat, annihilation of the threat is only one of the defence strategies available to ensure survival. In some cases, attempted annihilation may do more harm than good through collateral damage to host tissues. Other than this traditional concept of ‘resistance’ the other strategies available to the threatened potential host include ‘avoidance’ of the threat prior to infection as well as ‘tolerance’ (28). In the lung, the mechanisms of resistance (e.g., the innate and adaptive immune responses) and avoidance (e.g., nasal hairs, the cough reflex and the mucociliary escalator) are well described, but the concept of tolerance is poorly studied. Nevertheless, we see examples of tolerance in our thoracic clinics every day.
For instance, patients with CF live for decades with a microbial load in the lower respiratory tract that would probably be fatal to most hosts. Tolerance to infection, or so called ‘disease tolerance’ does not represent a failed eradication effort, but rather a highly specific host defence strategy which minimises the negative health effects of infection, without directly affecting pathogen burden (28). Chronic Pseudomonas infection in the CF lung is an example and is associated with classic features of the tolerant state, including tolerance to endotoxin (29) and polarisation of circulating monocytes toward an M2 phenotype. These cells are characterised not only by reduced secretion of pro-inflammatory cytokines like TNFα and secretion of anti-inflammatory cytokines like IL-10, but also an impaired capacity to present antigen despite markedly enhanced phagocytic activity (29). Recently, as would be predicted from the disease tolerance model, multiple investigators have noted that host health is dissociated from Pseudomonas biomass in CF (30-32), and similar observations have been made in lung transplant recipients (33). Recently, and again in the setting of lung transplantation, we have demonstrated that tolerance to Pseudomonas translates into allograft tolerance (Figure 1) (34). It is likely that gaining a better understanding of the tolerogenic mechanisms in operation at the interface of the airway mucosa and the role of commensal bacteria will provide important insights into COPD pathogenesis, and potentially guide the development of new treatments. This will depend heavily on accurately describing commensal microbial ecology in the COPD lung, an aim now achievable through the application of metagenomic techniques.
The microbiome in COPD—is it only about the lung?
Most microbiome studies to date have involved the gastrointestinal (GI) tract and diseases at that site such as colitis. Yet it is increasingly being recognised that changes to the GI microbiome can have profound effects on extraintestinal organs.
One of the earliest observations has been the effect of antibiotics on obesity. It has long been known that antibiotics can lead to increased weight gain in farm animals and have been used as growth factors in standard practice in many countries since the 1950s (35). This observation has been closely investigated and links have been shown between antibiotic use and obesity in mice (36), and with weight gain in malnourished humans (37). Metagenomics of the gut microbiome in murine studies has revealed that antibiotics cause a shift in microbial populations to those capable of producing short chain fatty acids as metabolites. These metabolites have been associated with improvements to colonic and systemic health. Furthermore the antibiotics caused an upregulation of liver enzymes involved in lipogenesis and triglyceride synthesis (36). Other studies have demonstrated gut microbiome effects on diabetes and atherosclerosis through alterations in the metabolites produced by the microbes that mediate communication between the microbiome and its host (38,39).
To date, the involvement of the microbiota in lung health has only been inferred through associative studies. There are no studies that demonstrate a direct effect of changes to the lung microbiome causing a subsequent change to lung health. This is in part due to the field’s infancy, and because the microbial load in the lung is low and the potential for contamination with microbes from the oral cavity and/or nasopharynx during sampling is high. However, studies are emerging that highlight the involvement of the gut microbiome in maintaining lung health and in contributing to lung disease. Ichinohe et al., demonstrated that mice treated with oral antibiotics had diminished immune responses when subsequently infected with influenza (40). Specifically, treated mice had significant reduced influenza antibody titres and CD4 and CD8 T-cell responses. In another study, Russell et al., demonstrated that neonatal mice given oral vancomycin had increased immune responses in the lung when challenged with ovalbumin in a model of allergic asthma. These mice also had increased airway hyperresponsiveness compared to untreated mice (41). Complementary to this, Ong et al., were able to demonstrate a strong association in children given antibiotics in the first year of life with the development of both transient and persistent asthma. These associations were strong even when children who received antibiotics for respiratory tract infections were excluded (42).
In regard to COPD, it has been known for many years that smoking impacts intestinal as well as lung health (43). Furthermore, there is a strong association between inflammatory bowel diseases and COPD, with many patients are also affected with Crohn’s disease (44,45). Moreover, smoking and smoking cessation have been recently shown to have clear effects on the microbiome of the gut (46). Thus, the evidence suggests that smoking can affect gut microbiota which in turn may induce systemic effects.
Conclusions & future directions
Since bacterial colonisation and infection is common in COPD and is central to the pathogenesis of exacerbations, gaining a more comprehensive understanding of lower respiratory tract bacterial ecology in patients with COPD is likely to be of considerable importance. The tools to achieve this objective are now readily available, but in order to make sense of the findings in stable COPD, the more fundamental question ‘do we have a normal lower respiratory tract microbiota?’ will need to be answered. It is also apparent that accurate determination of the makeup of the lower respiratory tract microbiome is confounded in studies where access to the lower respiratory tract is gained via the oropharynx where the bacterial biomass is high, even if a protected brush is used (47). Animal studies and approaches to obtaining human material surgically (e.g., at the time of lung resection and transplantation) can circumvent this difficulty. In the future, determining the makeup of the microbiome in healthy smokers and patients with mild COPD will assist in determining whether dysbiosis is a triggering event for COPD progression or whether it is a biomarker of more severe disease. Furthermore, it is likely that non-bacterial microbes will contribute to COPD pathogenesis, so determining the makeup of the viral and fungal microbiomes (the ‘virome’ and ‘mycobiome’), alongside the bacterial microbiome, in COPD will also be key objectives. These studies will complement, and may, in terms of their impact on the practice of medicine, even outshine the findings of the genomic era which began with the sequencing of the human genome (48).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Olsen GJ, Lane DJ, Giovannoni SJ, et al. Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol 1986;40:337-65. [PubMed]
- Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 1998;180:4765-74. [PubMed]
- Bond PL, Hugenholtz P, Keller J, et al. Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludges from sequencing batch reactors. Appl Environ Microbiol 1995;61:1910-6. [PubMed]
- Tringe SG, Hugenholtz P. A renaissance for the pioneering 16S rRNA gene. Curr Opin Microbiol 2008;11:442-6. [PubMed]
- Hugenholtz P, Tyson GW. Microbiology: metagenomics. Nature 2008;455:481-3. [PubMed]
- Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell 2012;148:1258-70. [PubMed]
- Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 2011;184:957-63. [PubMed]
- Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 2011;6:e16384. [PubMed]
- Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One 2010;5:e8578. [PubMed]
- Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:1073-80. [PubMed]
- Beck JM, Young VB, Huffnagle GB. The microbiome of the lung. Transl Res 2012;160:258-66. [PubMed]
- Dickson RP, Erb-Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med 2014;2:238-46. [PubMed]
- Ley RE, Lozupone CA, Hamady M, et al. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol 2008;6:776-88. [PubMed]
- Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027-31. [PubMed]
- Pragman AA, Kim HB, Reilly CS, et al. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One 2012;7:e47305. [PubMed]
- Zakharkina T, Heinzel E, Koczulla RA, et al. Analysis of the airway microbiota of healthy individuals and patients with chronic obstructive pulmonary disease by T-RFLP and clone sequencing. PLoS One 2013;8:e68302. [PubMed]
- Millares L, Ferrari R, Gallego M, et al. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 2014;33:1101-11. [PubMed]
- Huang YJ, Kim E, Cox MJ, et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS 2010;14:9-59. [PubMed]
- Huang YJ, Sethi S, Murphy T, et al. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 2014;52:2813-23. [PubMed]
- Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax 2006;61:250-8. [PubMed]
- Beckett EL, Stevens RL, Jarnicki AG, et al. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J Allergy Clin Immunol 2013;131:752-62. [PubMed]
- Franklin BS, Bossaller L, De Nardo D, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 2014;15:727-37. [PubMed]
- Starkey MR, Jarnicki AG, Essilfie AT, et al. Murine models of infectious exacerbations of airway inflammation. Curr Opin Pharmacol 2013;13:337-44. [PubMed]
- Hansbro PM, Hamilton MJ, Fricker M, et al. Importance of mast cell Prss31/transmembrane tryptase/tryptase-γ in lung function and experimental chronic obstructive pulmonary disease and colitis. J Biol Chem 2014;289:18214-27. [PubMed]
- Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013;68:1029-36. [PubMed]
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89. [PubMed]
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355-65. [PubMed]
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science 2012;335:936-41. [PubMed]
- Pena OM, Pistolic J, Raj D, et al. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol 2011;186:7243-54. [PubMed]
- Fodor AA, Klem ER, Gilpin DF, et al. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One 2012;7:e45001. [PubMed]
- Stressmann FA, Rogers GB, Marsh P, et al. Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation? J Cyst Fibros 2011;10:357-65. [PubMed]
- Zhao J, Schloss PD, Kalikin LM, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A 2012;109:5809-14. [PubMed]
- Hodge G, Hodge S, Reynolds PN, et al. Airway infection in stable lung transplant patients is associated with decreased intracellular T-helper type 1 pro-inflammatory cytokines in bronchoalveolar lavage T-cell subsets. Transpl Infect Dis 2008;10:99-105. [PubMed]
- Willner DL, Hugenholtz P, Yerkovich ST, et al. Reestablishment of recipient-associated microbiota in the lung allograft is linked to reduced risk of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2013;187:640-7. [PubMed]
- Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin Microbiol Rev 2003;16:175-88. [PubMed]
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621-6. [PubMed]
- Trehan I, Goldbach HS, LaGrone LN, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 2013;368:425-35. [PubMed]
- Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242-9. [PubMed]
- Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest 2014;124:4204-11. [PubMed]
- Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 2011;108:5354-9. [PubMed]
- Russell SL, Gold MJ, Hartmann M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 2012;13:440-7. [PubMed]
- Ong MS, Umetsu DT, Mandl KD. Consequences of antibiotics and infections in infancy: bugs, drugs, and wheezing. Ann Allergy Asthma Immunol 2014;112:441-445.e1.
- Birrenbach T, Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis 2004;10:848-59. [PubMed]
- Ekbom A, Brandt L, Granath F, et al. Increased risk of both ulcerative colitis and Crohn's disease in a population suffering from COPD. Lung 2008;186:167-72. [PubMed]
- Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012;5:7-18. [PubMed]
- Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One 2013;8:e59260. [PubMed]
- Kirkpatrick MB, Bass JB Jr. Quantitative bacterial cultures of bronchoalveolar lavage fluids and protected brush catheter specimens from normal subjects. Am Rev Respir Dis 1989;139:546-8. [PubMed]
- Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature 2001;409:860-921. [PubMed]


