The association of lipopolysaccharide and inflammatory factors with hepatopulmonary syndrome and their changes after orthotopic liver transplantation
Introduction
Hepatopulmonary syndrome (HPS) is a relatively common and severe pulmonary vascular complication of advanced liver cirrhosis and/or portal hypertension (PH), occurring in 10-30% of patients with cirrhosis (1). It is characterized by pulmonary microvascular dilatation and remodeling, resulting in impaired oxygenation in the absence of marked intrinsic cardiopulmonary disease (2,3). Medical therapy for severe HPS has generally been ineffective. Orthotopic liver transplantation (OLT) is the only successful treatment and typically results in complete resolution of the gas exchange impairment (4-8). However, the pathogenesis of HPS is unknown. Inflammation might play a role in its pathogenesis and development (9). The objectives of our study were to investigate the role of lipopolysaccharide (LPS), toll-like receptor 2 (TLR2), inducible nitric oxide synthase (iNOS), tissue necrosis factor alpha (TNF-α) and endothelin-1 (ET-1) in HPS and the change in the levels of these factors after OLT.
Materials and methods
Patient selection
From March 2004 to January 2006, 279 patients with end-stage liver disease received OLT in the Liver Transplantation Center of Sun Yat-sen University. During this period, 31 patients with HPS were evaluated in our center and were included in this analysis. Among these 31 HPS patients, 26 underwent OLT and the other five patients did not undergo OLT due to either late timing or expectation of a poor outcome. Written informed consent was obtained from each patient included in the study. The study protocol was approved by the institution’s Human Research Committee and the Medical Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University. Ten other healthy volunteers were selected as the healthy control group.
Diagnostic criteria for HPS
HPS was diagnosed by: (I) evidence of chronic liver disease with clinical manifestations of PH (varices, ascites, splenomegaly); (II) abnormal arterial oxygenation (PaO2 ≤70 mmHg) while breathing room air; and (III) “positive” 99m technetium macroaggregated albumin (99mTc-MAA) lung and brain perfusion scans suggesting intrapulmonary vascular dilatation (abnormal pulmonary shunt >7%), or positive contrast echocardiography. Non-HPS patients were those with end-stage liver disease without hypoxemia (PaO2 ≥70 mmHg) (10).
99mTc-MAA lung perfusion scan
Every patient with PaO2 ≤70 mmHg underwent a 99mTc-MAA lung and brain perfusion scan to specifically quantify the degree of intrapulmonary vascular dilation (11). Twenty minutes after injection (standing) of 2 mCi of 99mTc-MAA with 90% of particles between 10 and 90 µm (Dupont Pulmolite, Billerica, MA, USA), quantitative brain imaging was performed in the supine position.
Brain uptake or shunt fraction (assuming a constant 13% blood flow to the brain) was obtained by the following calculation: brain uptake (%) = (GMT brain/0.13)/(GMT brain/0.13+ GMT lung). GMT is the geometric mean count of 99mTc-MAA in the brain and lung. An abnormal intrapulmonary shunt was defined as greater than 7%. The severity of liver disease was described by the Child-Turrcote-Pugh (CTP) classification (A, B, or C) and the Model for End-stage Liver Disease (MELD) score. These variables were assessed within one week of the PaO2 measurements.
Blood sample collection
Fasting venous blood samples from all OLT patients with HPS were collected on the morning before transplantation and on postoperative days 3, 7, 14, 21 and 28. Samples were also taken from HPS patients without OLT once, and from healthy volunteers once for controls. Peripheral blood levels of TLR2 messenger ribonucleic acid (mRNA), iNOS mRNA, LPS, TNF-α and ET-1 were measured. Liver function and arterial blood gas (ABG) was examined periodically.
Experimental procedure
The expression levels of TLR2 mRNA and iNOS mRNA were measured by the fluorogenic quantitative polymerase chain reaction (FQ-PCR). The instrument, reagents and technique for real time fluorogenic quantitative reverse transcription-PCR (RT-PCR) were provided by Da An Gene Co., Ltd. of Sun Yat-Sen University. The following procedure was used: (I) peripheral blood total RNA extraction: total RNA was extracted from 500 µL of EDTA-anticoagulated whole blood cells and stored at –80 °C with ethanol for preservation; (II) primers and probe: the primer sequence of TLR2 was the forward primer 5'-CATTCCCTCAGGGCTCACAG-3' and the reverse primer 5'-TTGTTGGACAGGTCAAGGCTT-3'; the primer sequence of iNOS was the forward primer 5'-AATGGCTGGTACATGGGCAC-3' and the reverse primer 5'-GACGTCA CAGAAGTCCCGGA-3'; (III) reverse transcription: for reverse transcription, 4 µL of extracted RNA were used with the Qiagen RT-PCR Kit (Qiagen, Venlo, Netherlands) in the Perkin Elmer PE 9700 PCR instrument (Perkin Elmer, Waltham, MA). The reaction was conducted using 4 µL of RT-PCR buffer, 0.4 µL (10 pmol/µL) of forward primers, 0.4 µL (10 pmol/µL) of reverse primers, 0.2 µL (25 mmol/L) of dNTP Mix, l µL (10 U/µL) of MMLV, 10 µL of RNase-free water, and 4 µL of total RNA in a final volume of 20 µL. The RT-PCR buffer contains the combination of 50 mmol/L of Tris-HCl (pH 8.0), 50 mmol/L of KCl, 4 mmol/L of MgCl2, and 10 mmol/L of DTT. Reaction conditions were an initial incubation at 37 °C for one hour, followed by 95 °C for 3 min; (IV) positive control cDNA template preparation and quantitative polymerase chain reaction (Q-PCR): for Q-PCR, 5 µL of the RT-PCR cDNA products were used in the Perkin Elmer PE9700 PCR instrument. Reverse and positive control cDNA were in a mixture as follows: 10 µL of 5× SYBR Green 1 buffer, 1 µL (10 pmol/µL) of forward primers, 1 µL (10 pmol/µL) of reverse primers, 0.5 µL (25 mmoL/L) of dNTPs, 1.5 µL (2 U/µL) of Taq DNA Polymerase, 5 µL of cDNA, 31 µL of RNase-free water up to a final volume of 50 µL. Initial reactions at 94 °C for three min were followed by 40 cycles of 93 °C for 1 min and 55 °C for 1 min, and 72 °C for 1 min. Final results were automatically analyzed by the computer.
LPS was detected by the EDS-99 LPS detection system (Jinshanchuan Co. Ltd., Beijing) through a dynamic turbidimetric method. TNF-α and ET-1 were detected by Elisa methodology.
Statistical analysis
Patient demographic data are described by the mean and standard deviation for quantitative variables and percentages for binomial variables. SPSS 10.0 software was used for statistical analysis. Measurement data were analyzed by a t-test and expressed as mean ± standard deviation. P<0.05 was considered statistically significant.
Results
Patient characteristics
From March 2004 through January 2006, 31 with end-stage liver disease were diagnosed with HPS. Twenty-eight (90%) were male and three (10%) were female, and the mean age was 48 years (range, 28-69 years). Among the 31 HPS patients, 26 (84%) underwent OLT, and five potential OLT candidates (16%) did not undergo transplantation. All patients met minimal listing criteria for liver transplantation (CTP score ≥7 points). The clinical data of 26 OLT recipients including gender ratios, average age, and etiologies of liver cirrhosis, as well as MELD and CTP scores before OLT were listed in Table 1.
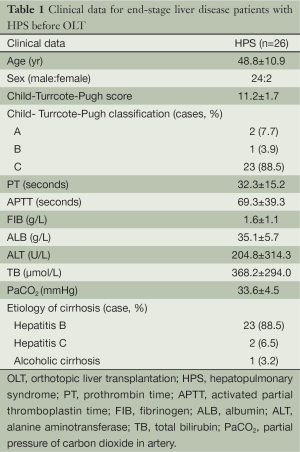
Full table
Levels of inflammatory factors in HPS and healthy control groups
The expression levels of TLR2 mRNA and iNOS mRNA and the plasma levels of LPS, TNF-α and ET-1 before operation are shown for the HPS and healthy control groups in Table 2.
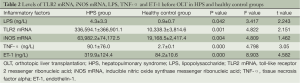
Full table
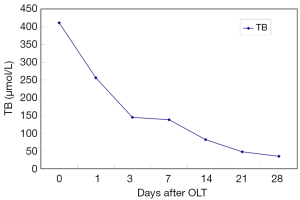
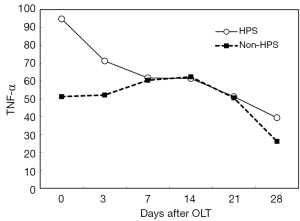
After OLT, liver function improved greatly. The decrease of total bilirubin (TB) is shown in Figure 1. In all HPS recipients, the levels of TLR2 mRNA (Figure 2), TNF-α (Figure 3), and ET-1 (Figure 4) decreased 28 days after OLT.
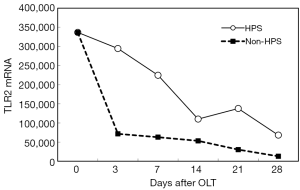
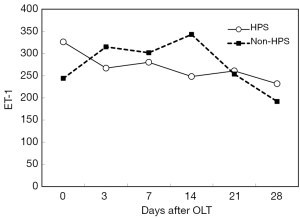
Liver function, oxygenation, and intrapulmonary shunt improved after OLT
PaO2 and PaO2/FiO2 in HPS patients improved to normal within 28 days after OLT with the normalization of liver function (Table 3). The intrapulmonary shunt measured by 99mTc-MAA decreased to less than 7% when the PaO2/FiO2 recovered to normal.

Full table
Discussion
HPS is a common complication in advanced liver disease patients. In our research, we proved that LPS increased in end-stage liver disease and was especially significant in those with HPS. This means that LPS might play a role in the pathogenesis of HPS. We concluded that the increased LPS might come from the intestine because the intestine is the biggest reservoir for intestinal bacteria and endotoxin, and the intestinal barrier is damaged severely in cirrhosis (12). PH and impaired microvascular circulation of the intestinal mucosa are associated with decreased intestinal movement, poor repair of the damaged barrier, and bacterial overgrowth, resulting in bacterial imbalance and dislocation because of increased permeability of the intestinal barrier. In cirrhosis, the incidence of intestinal bacterial dislocation was 48% (13,14). Bacteria and endotoxin from the intestine were released into the circulation, resulting in endotoxemia and inflammation with the release of various inflammatory cytokines (14).
In our research, we found that the levels of LPS, TLR2 mRNA, iNOS mRNA, TNF-α and ET-1 were significantly higher in the HPS group before OLT than in the healthy control group (P<0.05). Our results demonstrated that LPS and inflammatory cytokines, such as TNF-α, ET-1 and NO, might play a role in the pathogenesis and pathophysiology of HPS through TLR2. TLR2 (15), part of the transmembrane protein TLR family, was expressed by antigen-presenting cells and cells of the innate immune system, including neutrophils, mastocytes, basophils and eosinophilic granulocytes, as well as those exposed to the external environment, such as epithelial and endothelial cells in the lung and the gastrointestinal tract. TLR2 is essential for the recognition of a variety of pathogen-associated molecular patterns (PAMPs) from gram-positive bacteria, including bacterial lipoproteins, LPS, lipomannans and lipoteichoic acids. These highly conserved, soluble, membrane-bound proteins are collectively called pattern-recognition receptors (PRRs), and it is the PAMP/PRR interaction that triggers the innate immune system (16). LPS is a glycolipid constituent of the outer membrane of gram-negative bacteria, bound to LPS-binding proteins (LBP). With CD14 and MD-2, the LPS-LBP complex combines with TLR4/TLR2 and initiates the TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-κB signaling cascade in cells, similar to the process followed by TLR4 with a similar downstream effect. Finally, NF-κB is activated and causes the release of IL-12, IFN-γ, TNF-α, IL-1, IL-6, NO, ET-1 (17). ET-1 is released by Kupffer cells in the liver and macrophages in the lungs. Its level increases greatly because of liver dysfunction and collateral circulation, and through the ETB receptor, it activates eNOS (18,19), causing accumulation of macrophages in the pulmonary vasculature and overexpression of iNOS and HO-1. The overproduction of NO and carbon monoxide (CO) results in widespread pulmonary vasodilatation at the precapillary and capillary level that leads to a right-to-left shunt, pulmonary V/Q unbalance and hypoxemia.
On the other hand, with the improvement of liver function in HPS patients, TLR2 mRNA, TNF-α and ET-1 decrease, and then, hypoxemia is relieved gradually. In our study, the decrease of these inflammatory factors did not reach statistical significance at 28 days after OLT; however, longer observation might be needed. LPS and iNOS mRNA decreased early after OLT, but they increased again later. HPS patients are extremely prone to the complication of pneumonia. We hypothesized that a later increase in LPS and iNOS mRNA might be associated with subsequent infection in some HPS patients.
Our research demonstrated that LPS might upregulated TLR2 mRNA expression and activation of the downstream inflammatory factor production in cirrhosis before OLT. The overproduction of inflammatory factors might play an important role in the pathogenesis and pathophysiology of HPS. We supposed that LPS came from the intestine because of a damaged intestinal barrier in cirrhosis. After OLT, the liver and intestinal function recovered, and the intestinal barrier was repaired; thus, the levels of inflammatory factors decreased as the hypoxemia of HPS was relieved gradually and completely.
There were limitations in our research in that we could not prove that the origin of LPS was from the intestine. Nor did we detect the expression level of TLR4, which was predominantly activated by LPS. Further research is needed to clarify the function of immune cells and how they work through LPS and TLR activation in HPS.
Conclusions
Above all, LPS and inflammatory factors increased at the end stage of liver disease before OLT, but decreased after OLT with the recovery of liver function. Oxygenation of lungs also got better and the HPS was cured. Our results showed LPS with the release of series of inflammatory factors may be associated with the pathogenesis and development of HPS.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Grace JA, Angus PW. Hepatopulmonary syndrome: update on recent advances in pathophysiology, investigation, and treatment. J Gastroenterol Hepatol 2013;28:213-9. [PubMed]
- Agarwal PD, Hughes PJ, Runo JR, et al. The clinical significance of intrapulmonary vascular dilations in liver transplant candidates. Clin Transplant 2013;27:148-53. [PubMed]
- Zhang J, Luo B, Tang L, et al. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology 2009;136:1070-80. [PubMed]
- Yi HM, Chen GH, An YL, et al. Effect of orthotopic liver transplantation on hepatopulmonary syndrome: 5 years of postoperative follow-up and survival analysis. Organ Transplantation 2012;03:145-50.
- Yi HM, Wang GS, Yi SH, et al. Prospective evaluation of postoperative outcome after liver transplantation in hepatopulmonary syndrome patients. Chin Med J (Engl) 2009;122:2598-602. [PubMed]
- Houlihan DD, Holt A, Elliot C, et al. Review article: liver transplantation for the pulmonary disorders of portal hypertension. Aliment Pharmacol Ther 2013;37:183-94. [PubMed]
- Saigal S, Choudhary N, Saraf N, et al. Excellent outcome of living donor liver transplantation in patients with hepatopulmonary syndrome: a single centre experience. Clin Transplant 2013;27:530-4. [PubMed]
- Gupta S, Castel H, Rao RV, et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant 2010;10:354-63. [PubMed]
- Liu L, Liu N, Zhao Z, et al. TNF-α neutralization improves experimental hepatopulmonary syndrome in rats. Liver Int 2012;32:1018-26. [PubMed]
- Perkins JD. Diagnosing hepatopulmonary syndrome. Liver Transpl 2007;13:1464-5. [PubMed]
- Abrams GA, Nanda NC, Dubovsky EV, et al. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology 1998;114:305-10. [PubMed]
- Cartin-Ceba R, Swanson KL, Krowka MJ, et al. Pulmonary arteriovenous malformations. Chest 2013;144:1033-44. [PubMed]
- Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome—a liver-induced lung vascular disorder. N Engl J Med 2008;358:2378-87. [PubMed]
- Sztrymf B, Libert JM, Mougeot C, et al. Cirrhotic rats with bacterial translocation have higher incidence and severity of hepatopulmonary syndrome. J Gastroenterol Hepatol 2005;20:1538-44. [PubMed]
- Riordan SM, Skinner N, Nagree A, et al. Peripheral blood mononuclear cell expression of toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology 2003;37:1154-64. [PubMed]
- Paik YH, Schwabe RF, Bataller R, et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003;37:1043-55. [PubMed]
- He H, Genovese KJ, Nisbet DJ, et al. Profile of Toll-like receptor expressions and induction of nitric oxide synthesis by Toll-like receptor agonists in chicken monocytes. Mol Immunol 2006;43:783-9. [PubMed]
- Showpittapornchai U, Wattanasirichaigoon S, Pradidarcheep W. Predominant vascular dilatation with NOS expression in lung lower lobe of thioacetamide induced-cirrhotic rat. J Med Assoc Thai 2012;95 Suppl 12:S99-104. [PubMed]
- Fallon MB, Zhang J. The lung in liver disease: old problem, new concepts. Trans Am Clin Climatol Assoc 2013;124:250-62. [PubMed]


