Implementing the new IASLC/ATS/ERS classification of lung adenocarcinomas: results from international and Chinese cohorts
Introduction
Lung cancer is the leading cause of cancer death worldwide (1). Over the past decade, the rate of adenocarcinoma (the most frequent subtype of lung cancer) has increased in most countries (2,3). Currently, the single most important factor that determines prognosis for patients with lung adenocarcinomas is tumor-nodal-metastasis stage (4). Lung adenocarcinoma is a heterogeneous tumor with variation in pathological profile. Histologic classifications of lung cancers have been published by the World Health Organization (WHO) in 1967, 1981, 1999, and 2004, and the most recent revision has introduced relevant clinical and genetic information (5). Despite this, there is still limited clinical utility in the 2004 classification of lung adenocarcinomas since more than 90% of adenocarcinomas are classified as a mixed subtype even though they have a variety of clinical outcomes (6-8). Increasing evidence suggests that histologic patterns can identify significant prognostic subsets of patients with lung adenocarcinomas (8-13). Multiple studies have shown that patients with pure lepidic (noninvasive) adenocarcinomas had 100%, 5-year disease-free survival (14-17). Other studies showed that patients with lepidic predominant, minimally invasive (≤5 mm invasion) adenocarcinomas had a near 100% survival (9,10,18). Lepidic predominant invasive tumors also correlate with a favorable prognosis in patients with resected lung adenocarcinomas (19-21). In contrast, the micropapillary pattern has been identified as a poor prognostic factor in patients with lung adenocarcinomas (22,23). To address the advances in the prognostic pathological findings identified over the last decade, a new histologic classification is needed to provide histological subtypes with uniform terminology and diagnostic criteria.
In addition to the pathologic findings that can define prognosis, there have been advances in radiologic-pathologic correlations, molecular biology, and thoracic medical oncology for lung adenocarcinomas over the past decade. On chest computed tomography (CT) of lung adenocarcinomas, the correlations between lepidic growth and ground-glass opacities, and between invasive components and solid components, have been identified and used for predicting histologic subtypes and patient prognosis. CT has also been used for improving preoperative clinical decision-making of surgical procedures (i.e., lobectomy vs. limited resection) (24-27).
Recent advances in molecular biology, in partner with medical oncology advances, have shown that activating mutations in the tyrosine kinase domain of epidermal growth factor receptors (EGFR) can predict better responsiveness to EGFR tyrosine kinase inhibitors (TKI) than conventional platinum-based chemotherapy in patients with non-small cell lung cancer (NSCLC) (28-32). These mutations are most frequently observed in females, in never smokers, and in Asian patients with adenocarcinomas (28-34). EGFR mutations have also been associated with lepidic pattern adenocarcinomas, formerly known as a bronchioloalveolar carcinoma patterns (34-39). This association has led to the hypothesis that tumors with lepidic pattern adenocarcinomas may be correlated with the EGFR mutations and may predict responses to TKI (40-42). In contrast, Kirsten rat sarcoma viral oncogene homolog (KRAS) and v-raf murine sarcoma viral oncogene homolog B (BRAF), the downstream molecules in the EGFR signaling pathway, were considered resistant to EGFR-TKI treatment and exhibited poor prognosis (34,43-50). In addition, the KRAS mutation has shown a correlation with invasive mucinous adenocarcinomas, formerly known as mucinous bronchioloalveolar carcinomas (51-55). A recently discovered anaplastic lymphoma kinase (ALK) rearrangement can predict responsiveness to a new targeted agent (crizotinib) (56-58). ALK rearrangements exclusively occur in lung adenocarcinomas and they are correlated with specific histological findings such as signet-ring cell features, extracellular mucin, and cribriform patterns (59-61).
History of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) histologic classification of lung adenocarcinoma
To provide an international and multidisciplinary approach to the development of a new histologic classification system for identifying prognostic subtype, the IASLC/ATS/ERS selected as panel members thoracic medical oncologists, pulmonologists, radiologists, molecular biologists, thoracic surgeons, and pathologists based on their special interest and expertise in lung adenocarcinomas (6). First, the panel performed a systematic review of the literature on lung adenocarcinomas and generated a series of key questions by specialty. The search strategy initially yielded 11,368 relevant articles. Of these, 312 met the specified eligibility criteria for a full-text review. After review, and in conjunction with each specialty group, a writing committee developed the recommendations for histologic classification. Following a multidisciplinary discussion that took place between 2008 and 2009, this classification system was subsequently modified, and separate projects were initiated by the panel members in an effort to validate the proposed system (7,11,62). On the basis of this multidisciplinary approach, the panel recommended 10 significant changes to the diagnostic classification of lung adenocarcinomas in order to improve precision in predicting clinical outcome and therapeutic benefits. These recommendations are detailed in the 2011 joint publication by the IASLC, ATS, and ERS proposing the new classification system (6).
The 2011 IASLC/ATS/ERS lung adenocarcinoma histologic classification and advantage
The IASLC/ATS/ERS lung adenocarcinoma histologic classification system was proposed in the Journal of Thoracic Oncology in 2011 (6). According to this new classification, tumor size ≤3 cm with pure lepidic pattern, but without lymphatic, vascular, pleural invasion or tumor necrosis was defined as adenocarcinoma in situ (AIS). If tumor size ≤3 cm with a lepidic predominant pattern and contained ≤5 mm stromal invasion it was defined as minimally invasive adenocarcinoma (MIA). If tumor had >5 mm stromal invasion it was defined as an invasive adenocarcinoma. For invasive adenocarcinomas, comprehensive histologic subtyping by recording the percentage of each histological component (lepidic, acinar, papillary, micropapillary, or solid) in 5% increments is suggested to choose a single predominant pattern. Variant invasive adenocarcinomas included invasive mucinous adenocarcinomas (formerly mucinous bronchioloalveolar carcinomas), colloid, enteric, and fetal (low and high grade) adenocarcinomas.
This new classification provides not only uniform terminology and diagnostic criteria for pathologists, but it is also predictive and it provides prognostic data that may help oncologists, thoracic surgeons, and radiologists improve patient outcomes (Table 1).
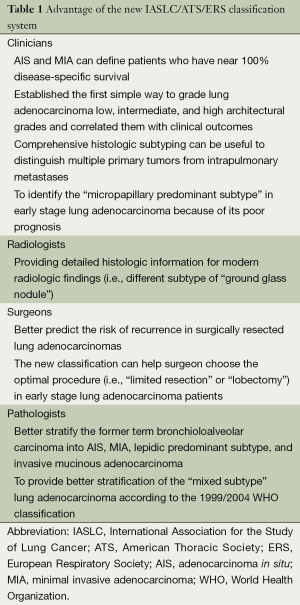
Full table
Validation and implementing studies of the 2011 IASLC/ATS/ERS lung adenocarcinoma histologic classification
Current international cohorts validate the IASLC classification had prognostic value
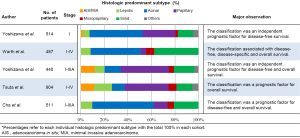
A review of five published studies that validated the 2011 IASLC/ATS/ERS lung adenocarcinoma histological classification using a large cohort (more than 300 patients as a study sample) is shown in Figure 1 (7,63-66). The study cohorts consisted of one from Unites States, Germany, and South Korea, and two from Japan. All five studies included patients who underwent curative-intent surgery. The study from the United States validated the new classification by using a homogeneous cohort composed of only stage I patients (7) while the other studies from Japan, Germany, and South Korea used patients with both early- and advanced-stage lung adenocarcinomas (63-66). The majority of cases (48-78%) seen globally were adenocarcinomas of the acinar and papillary predominant histologic subtypes. Based on the new IASLC/ATS/ERS classification, five of these large cohorts demonstrated the significant difference between clinical prognoses among the five predominant histologic subtypes. In addition, based on the similarity of survival rate, three prognostic groups with low, intermediate, and high architectural grades were proposed (7). The low grade was comprised of AIS and MIA, and it had a near 100% 5-year survival rate. The intermediate grade consisted of acinar and papillary predominant adenocarcinomas. High grade tumors included solid and micropapillary predominant adenocarcinomas and they presented with a poor outcome. Several additional cohorts also validated the prognostic difference between these three tumor grade groups (67-70).
The association between radiologist features of CT/positron emission tomography (PET)-CT and the IASLC classification
While there are many positive aspects of the new classification system, it is not without its limitations. One such limitation is that the histologic subtyping is primarily estimated using postoperatively resected specimens and not via preoperative small biopsies or cytology specimens (11). Therefore, it is preferable to use a preoperative surrogate biomarker in conjunction with imaging tools to predict patient prognosis. One such imaging tool is 18F-fluorodeoxyglucose-PET (FDG-PET), which is a standard imaging modality currently used in clinical practice. FDG-PET measures the metabolic activity of tumors and the maximum standardized uptake value (SUVmax) on FDG-PET has shown to correlate with prognosis in lung cancer patients (71-73). With this in mind, we investigated the association between the histologic predominant subtypes of the IASLC/ATS/ERS classification and SUVmax of PET. Our studies revealed that a high SUVmax correlated with high grade histologic subtypes in stage I lung adenocarcinomas. High SUVmax (≥3.0) was associated with a poor prognosis of recurrence and it could further stratify patients with intermediate architectural grade tumors (acinar or papillary predominant histologic subtypes) into two prognostic subsets (74). This result was validated by two other studies that showed that the presence of high architectural grade tumors (i.e., micropapillary and solid predominant histologic subtypes) were associated with a higher SUVmax value (66,75). These results may help clinicians to identify the patients with a higher preoperative risk of recurrence and assist them in selecting patients for neoadjuvant treatment or extended surgery.
The association between genetic mutation analysis and the IASLC/ATS/ERS classification
Following the aforementioned, genetic mutation variants are related to the treatment responsiveness of different targeting inhibitors. The identification of the correlation between histology and these molecular abnormalities becomes clinically relevant when choosing which patients will receive properly targeted cancer therapies and predict treatment responsiveness (76).
Currently, EGFR mutations occur most frequently in AIS, MIA, and lepidic predominant histologic subtypes; they are relatively rare in solid predominant lung adenocarcinomas. Conversely, KRAS mutations were associated with invasive mucinous adenocarcinomas and the solid morphologic pattern in lung adenocarcinomas (8,63,64,77-81). In identical studies, especially those that used East Asian patients, a higher incidence of EGFR mutations were observed in micropapillary pattern lung adenocarcinomas (64,82-85). However, the some studies did not show this correlation (77-79,86). The implementation of more clinical trials will be needed to investigate the long term benefits garnered from TKI treatment in patients with micropapillary lung adenocarcinomas.
Current validation for Chinese population
Ethnic differences in the epidemiology and the clinical behavior of lung cancer between East Asians and Caucasians have been acknowledged since the introduction of EGFR TKIs and the subsequent discovery of activating EGFR mutations (87,88). To understand the relationship between the new IASLC histologic subtyping classification and genetic mutations in the Chinese population, three studies were proposed. The EGFR mutation was positively associated with lepidic, acinar, and micropapillary predominant histologic subtypes and negatively associated with solid predominant histologic subtypes (77,84,85). In contrast, the KRAS mutation was positively associated with invasive mucinous adenocarcinomas (77,84). These results were similar to other studies conducted on Western populations except for two of them that showed that EGFR mutations positively correlated with the micropapillary histologic subtype (84,85). Further studies were warranted to confirm that there was a difference between EGFR mutation incidence rate of the micropapillary histologic subtype in East Asian and Caucasian populations.
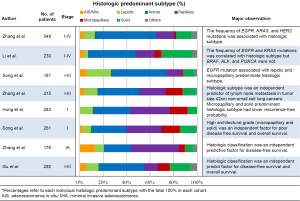
With the regard to the validation of the prognostic value of the new IASLC/ATS/ERS classification, there were five studies that demonstrated that the histologic subtypes were independent predictors for a patient’s clinical outcome (67-70,89). These results (shown in Figure 2) confirm that the new classification system will be applicable to the Chinese population and could be useful in the selection patients for personalized therapies.
The IASLC/ATS/ERS classification and surgical procedure options
We further investigated the prognostic significance of the histologic pattern in small (≤2 cm) stage I lung adenocarcinoma patients who underwent different surgical procedures (limited resection vs. lobectomy) (90). We saw that patients who had a micropapillary morphologic pattern of ≥5% and were treated with limited resection (wedge resection or segmentectomy) had a higher incidence of locoregional recurrence while those treated with lobectomy had a lower incidence locoregional recurrence. This suggests that this histologic pattern has a greater chance of locoregional recurrence in comparison to other histologic morphologies. It is the important to note that there was a reduced probability of recurrence in cases with a surgical margin of ≥1 cm. The results of our study suggest that patients treated with limited resection and whose tumors are determined to have a micropapillary of ≥5% (this is determined by the use of permanent sections) may require a complete lobectomy or further adjuvant treatment. Although limited resections had a higher locoregional recurrence rate in early stage I lung adenocarcinoma with micropapillary ≥5%, it might be useful to investigate the utility of extended surgeries, such as lobectomies, in those types of patients. However, identifying the presence of small percentage of micropapillary morphologic patterns on preoperative imaging, core biopsies and intraoperative frozen sections is difficult and unreliable (91,92). Further studies were warranted to overcome this condition.
Prognostic factors not included in the 2011 IASLC/ATS/ERS lung adenocarcinoma histologic classification
According to the aforementioned large cohort validation studies, the 2011 IASLC/ATS/ERS lung adenocarcinoma histologic classification has great prognostic value (7,63,64). In addition to this, we have recently published studies that discuss the use of several prognostic factors that are based on morphological analysis (histologic features such as nuclear feature, cribriform subtype, and presence of a micropapillary pattern), immunohistochemical analysis [Ki-67 labeling index and thyroid transcription factor-1 (TTF-1)], and immune markers (tumor-infiltrating lymphocyte and cytokine receptor expression), when investigating a large cohort comprised of stage I lung adenocarcinoma patients (90,93-96).
Using a cohort of stage I lung adenocarcinoma patients, we evaluated all of the nuclear features (nuclear diameter, nuclear atypia, nuclear/cytoplasmic ratio, chromatin pattern, prominence of nucleoli, intranuclear inclusions, mitotic count, and atypical mitoses) and identified nuclear diameter, nuclear atypia, mitotic count, and atypical mitoses as predictors of an increased risk of recurrence (93). Among these features, we discovered that mitotic count was an independent risk factor of recurrence. Using this information, we established a combined architectural (based on the 2011 IASLC/ATS/ERS classification) and mitotic count grading system. This new system was able to better stratify patients for risk of recurrence when compared with the stratification system used in the 2011 IASLC/ATS/ERS classification alone.
We reported the prognostic significance of the cribriform pattern as a predominant subtype. In conjunction with the 2011 IASLC/ATS/ERS classification, we proposed using the cribriform pattern as a distinct histologic subtype with a poor prognosis (94). The recurrence-free probability for patients with cribriform predominant tumors was significantly lower than it was for patients with acinar or papillary predominant tumors and comparable to patients with micropapillary or solid predominant tumors. These findings give credence to the hypothesis that the cribriform pattern was an independent prognostic factor.
In addition to mitotic count, Ki-67 also represents a proliferation of tumor cells. Based on immunohistochemical analysis using tissue microarrays on stage I lung adenocarcinomas, we reported a high Ki-67 labeling index; this was indicative of a predictor of recurrence (93). While TTF-1 is known as a positive diagnostic marker for differentiating between lung adenocarcinomas and squamous cell carcinomas, TTF-1 negativity is an independent risk factor of recurrence in stage I lung adenocarcinomas (95). More importantly, tumoral TTF-1 expression status was able to further stratify patients with intermediate grade tumors (acinar and papillary predominant subtype) based on their risk of recurrence.
Recent evidence suggests that the immune microenvironment has prognostic significance in solid cancers (97,98). We investigated the prognostic significance of tumor-infiltrating immune cells in tumor and tumor-related stroma, tumoral cytokine, and cytokine receptor expression via immunohistochemical analysis using tissue microarrays in two large, independent cohorts (training and validation; n=478 for each) of patients with stage I lung adenocarcinomas. We identified high forkhead box P3 (FoxP3)/CD3 lymphocyte infiltration ratio in tumor-related stroma, tumoral interleukin-7 receptor (IL-7R) overexpression, and a loss of IL-12Rβ2 expression as poor independent prognostic indicators of recurrence (96). All of these immune markers were able to further stratify the risk of recurrence in each histological grade based on the 2011 IASLC/ATS/ERS classification.
Future potential of the 2011 IASLC/ATS/ERS classification
Clinical trails comparing limited resections and lobectomies should also stratify patients according to these histologic architectural grades and morphologic subtypes. This is because patients with high architectural grade tumors (micropapillary and/or solid predominant subtypes) may be suitable for lobectomies while those with low grade tumors are more suitable for limited resections. The recent randomized trials that assessed low-dose CT screening for lung cancer (99-101) suggested that an increasing number of patients will be diagnosed with adenocarcinomas with lepidic growth at an early stage. This may ultimately contribute to a reduced disease-related mortality rate for those types of patients in the future. Therefore, it is important to recognize the clinical characterization of early-stage lung adenocarcinomas with lepidic predominant patterns. Since AIS and MIA are very curable, if completely resected, they have become of great interest to surgeons who may be considering limited resection over standard lobectomy as a treatment option.
While several previous clinical trials applied adjuvant chemotherapy to stage I NSCLC patients, that treatment yielded no clinical benefit (102,103). The 2011 IASLC/ATS/ERS classification identified patients in the high-risk group of recurrence such as those with micropapillary and solid predominant tumors. Additionally, the prognostic factors that we recently identified (nuclear grade, cribriform pattern, TTF-1 negativity, high Ki-67 labeling index, immune markers, and SUVmax on FDG-PET), provided better prognostic stratification than did the 2011 IASLC/ATS/ERS classification did alone (74,90,93-96). Therefore, we believe that the new classification system, which includes the previously mentioned factors, could help to identify stage I lung adenocarcinoma patients at a high-risk for recurrence who may benefit from adjuvant chemotherapy. This would, in turn, improve their overall survival rate.
The new IASLC/ATS/ERS adenocarcinoma classification may help in comparing histologic characteristics of synchronous multiple lung adenocarcinomas to determine whether they are intrapulmonary metastases or separate primaries. A combination of comprehensive histologic subtyping and other histologic characteristics have been shown to have a correlation with molecular analyses and clinical behavior (104,105).
The application of the new classification system in small specimens, including cytology, is still challenging and requires further investigation. In those small specimens, there may be other morphologic findings, such as nuclear grade (nuclear atypia and diameter), that could help stratify patients based on their risk of recurrence or cancer-related death (93,106).
Although the prognostic values of the 2011 IASLC/ATS/ERS classification have been validated, reproducibility (interobserver agreement) has not been adequately investigated to identify a predominant pattern in lung adenocarcinomas. The only way to confirm reproducibility and improve identification of each histologic pattern using this new classification system is through the development of more precise definitions combined with better training when interpreting system terminology.
Implications for Chinese physicians
With the number of diagnoses increasing and the need for management of lung cancer growing in the Chinese population, this new classification system is both timely and much needed. While this new classification system requires some training for pathologists interested in lung cancer, it can readily be implemented in any hospital that performs hematoxylin and eosin (H&E) staining. Furthermore, any H&E slide can easily be reviewed by pathologists at other treatment centers to confirm the diagnosis. Unlike molecular testing, which requires complex resources and advanced equipment, the IASLC/ATS/ERS classification system is easy to implement and has low maintenance costs. Awareness of this new classification system and the appropriate collaboration with high-volume centers for validation of the predominant histological subtype on H&E slides will assist treating physicians in stratifying the prognoses of their patients. Of even greater consequence, there is the possibility that this new classification system may identify differences in Chinese patients’ histologic subtype. This will form the basis for a modified classification for lung cancer management in Chinese patients.
Summary
The 2011 IASLC/ATS/ERS classification system has been proven to have powerful prognostic value in five large cohorts (>300 patients) across multiple countries (8,58-60). Patients with AIS and MIA had 100% DFS with no recurrent diseases. Patients with micropapillary or solid predominant tumors would be classified as a high-risk group for recurrence or cancer-related death. Patients with acinar predominant tumors will be classified as an intermediate risk group. Patients with papillary predominant tumors might be classified as an intermediate risk group, although further investigation would be needed. On the basis of our published studies, additional prognostic factors (nuclear grade, cribriform pattern, high Ki-67 labeling index, TTF-1 negativity, immune markers, and SUVmax on FDG-PET) and the 2011 IASLC/ATS/ERS classification system could further stratify patients into prognostic subgroups for recurrence and cancer-related death. Ultimately, this may aid in clinical management and decision making, especially for patients with early-stage lung adenocarcinomas, when deciding whether or not to opt for adjuvant chemotherapy.
This new classification system of lung adenocarcinomas, the predominant type of lung cancer, can be readily implemented at any hospital in China that has the capacity to perform H&E staining. The reproducibility of the classification system and its prognostic importance for patients with lung cancer in this setting require further investigation.
Acknowledgements
We thank Alex Torres for his editorial assistance.
Funding: This work was supported, in part, by William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center; the National Institutes of Health’s National Cancer Institute (R21 CA164568-01A1, R21 CA164585-01A1, U54 CA137788, R01 CA136705-06, P30 CA008748, and U54 CA132378); and the U.S. Department of Defense (LC110202 and PR101053).
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol 2008;3:819-31. [PubMed]
- Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005;117:294-9. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. eds. American Joint Committee on Cancer Cancer Staging Manual. 7th ed. New York, NY: Springer, 2009:253-70.
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. eds. World Health Organization Classification of Tumours. Pathology and Genetics. Tumours of the Lung, Pleura, Thymus, and Heart. Lyon, France: IARC Press, 2004.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [PubMed]
- Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008;32:810-27. [PubMed]
- Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol 2007;20:233-41. [PubMed]
- Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol 2009;33:462-9. [PubMed]
- Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010;34:1155-62. [PubMed]
- Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer 2010;116:659-69. [PubMed]
- Nakazato Y, Minami Y, Kobayashi H, et al. Nuclear grading of primary pulmonary adenocarcinomas: correlation between nuclear size and prognosis. Cancer 2010;116:2011-9. [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [PubMed]
- Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol 2004;28:198-206. [PubMed]
- Vazquez M, Carter D, Brambilla E, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009;64:148-54. [PubMed]
- Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg 2009;88:1106-11. [PubMed]
- Maeshima AM, Tochigi N, Yoshida A, et al. Histological scoring for small lung adenocarcinomas 2 cm or less in diameter: a reliable prognostic indicator. J Thorac Oncol 2010;5:333-9. [PubMed]
- Lee HY, Han J, Lee KS, et al. Lung adenocarcinoma as a solitary pulmonary nodule: prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer 2009;66:379-85. [PubMed]
- Yokose T, Suzuki K, Nagai K, et al. Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer 2000;29:179-88. [PubMed]
- Lin DM, Ma Y, Zheng S, et al. Prognostic value of bronchioloalveolar carcinoma component in lung adenocarcinoma. Histol Histopathol 2006;21:627-32. [PubMed]
- Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003;27:101-9. [PubMed]
- Nagano T, Ishii G, Nagai K, et al. Structural and biological properties of a papillary component generating a micropapillary component in lung adenocarcinoma. Lung Cancer 2010;67:282-9. [PubMed]
- Nakata M, Sawada S, Saeki H, et al. Prospective study of thoracoscopic limited resection for ground-glass opacity selected by computed tomography. Ann Thorac Surg 2003;75:1601-5; discussion 5-6. [PubMed]
- Suzuki K, Asamura H, Kusumoto M, et al. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635-9. [PubMed]
- Takashima S, Li F, Maruyama Y, et al. Discrimination of subtypes of small adenocarcinoma in the lung with thin-section CT. Lung Cancer 2002;36:175-82. [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [PubMed]
- Tam IY, Chung LP, Suen WS, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res 2006;12:1647-53. [PubMed]
- Miller VA, Hirsch FR, Johnson DH. Systemic therapy of advanced bronchioloalveolar cell carcinoma: challenges and opportunities. J Clin Oncol 2005;23:3288-93. [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [PubMed]
- Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005;23:857-65. [PubMed]
- Hsieh RK, Lim KH, Kuo HT, et al. Female sex and bronchioloalveolar pathologic subtype predict EGFR mutations in non-small cell lung cancer. Chest 2005;128:317-21. [PubMed]
- Blons H, Cote JF, Le Corre D, et al. Epidermal growth factor receptor mutation in lung cancer are linked to bronchioloalveolar differentiation. Am J Surg Pathol 2006;30:1309-15. [PubMed]
- Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol 2004;22:1103-9. [PubMed]
- Kim YH, Ishii G, Goto K, et al. Dominant papillary subtype is a significant predictor of the response to gefitinib in adenocarcinoma of the lung. Clin Cancer Res 2004;10:7311-7. [PubMed]
- Zakowski MF, Hussain S, Pao W, et al. Morphologic features of adenocarcinoma of the lung predictive of response to the epidermal growth factor receptor kinase inhibitors erlotinib and gefitinib. Arch Pathol Lab Med 2009;133:470-7. [PubMed]
- Ahrendt SA, Decker PA, Alawi EA, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer 2001;92:1525-30. [PubMed]
- Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2005;92:131-9. [PubMed]
- Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol 2008;3:111-6. [PubMed]
- De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA. Micropapillary lung adenocarcinoma: EGFR, K-ras, and BRAF mutational profile. Am J Clin Pathol 2009;131:694-700. [PubMed]
- Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 2007;98:1817-24. [PubMed]
- Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009;6:201-5. [PubMed]
- Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2005;2:e17. [PubMed]
- Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 2005;23:5900-9. [PubMed]
- Marchetti A, Buttitta F, Pellegrini S, et al. Bronchioloalveolar lung carcinomas: K-ras mutations are constant events in the mucinous subtype. J Pathol 1996;179:254-9. [PubMed]
- Finberg KE, Sequist LV, Joshi VA, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn 2007;9:320-6. [PubMed]
- Casali C, Rossi G, Marchioni A, et al. A single institution-based retrospective study of surgically treated bronchioloalveolar adenocarcinoma of the lung: clinicopathologic analysis, molecular features, and possible pitfalls in routine practice. J Thorac Oncol 2010;5:830-6. [PubMed]
- Hata A, Katakami N, Fujita S, et al. Frequency of EGFR and KRAS mutations in Japanese patients with lung adenocarcinoma with features of the mucinous subtype of bronchioloalveolar carcinoma. J Thorac Oncol 2010;5:1197-200. [PubMed]
- Kakegawa S, Shimizu K, Sugano M, et al. Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer 2011;117:4257-66. [PubMed]
- Sasaki T, Janne PA. New strategies for treatment of ALK-rearranged non-small cell lung cancers. Clin Cancer Res 2011;17:7213-8. [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009;22:508-15. [PubMed]
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216-23. [PubMed]
- Jokoji R, Yamasaki T, Minami S, et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol 2010;63:1066-70. [PubMed]
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [PubMed]
- Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [PubMed]
- Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: Clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 2014;147:921-928.e2.
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic Value of the New International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Lung Adenocarcinoma Classification on Death and Recurrence in Completely Resected Stage I Lung Adenocarcinoma. Ann Surg 2013;258:1079-86. [PubMed]
- Song Z, Zhu H, Guo Z, et al. Prognostic value of the IASLC/ATS/ERS classification in stage I lung adenocarcinoma patients-Based on a hospital study in China. Eur J Surg Oncol 2013;39:1262-8. [PubMed]
- Zhang J, Wu J, Tan Q, et al. Why Do Pathological Stage IA Lung Adenocarcinomas Vary from Prognosis?: A Clinicopathologic Study of 176 Patients with Pathological Stage IA Lung Adenocarcinoma Based on the IASLC/ATS/ERS Classification. J Thorac Oncol 2013;8:1196-202. [PubMed]
- Gu J, Lu C, Guo J, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 2013;107:474-80. [PubMed]
- Nakayama H, Okumura S, Daisaki H, et al. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: a multicenter study. Cancer 2010;116:3170-7. [PubMed]
- Nair VS, Barnett PG, Ananth L, et al. PET scan 18F-fluorodeoxyglucose uptake and prognosis in patients with resected clinical stage IA non-small cell lung cancer. Chest 2010;137:1150-6. [PubMed]
- Shiono S, Abiko M, Sato T. Positron emission tomography/computed tomography and lymphovascular invasion predict recurrence in stage I lung cancers. J Thorac Oncol 2011;6:43-7. [PubMed]
- Kadota K, Colovos C, Suzuki K, et al. FDG-PET SUVmax combined with IASLC/ATS/ERS histologic classification improves the prognostic stratification of patients with stage I lung adenocarcinoma. Ann Surg Oncol 2012;19:3598-605. [PubMed]
- Lee HY, Jeong JY, Lee KS, et al. Solitary pulmonary nodular lung adenocarcinoma: correlation of histopathologic scoring and patient survival with imaging biomarkers. Radiology 2012;264:884-93. [PubMed]
- Yoshida T, Ishii G, Goto K, et al. Solid predominant histology predicts EGFR tyrosine kinase inhibitor response in patients with EGFR mutation-positive lung adenocarcinoma. J Cancer Res Clin Oncol 2013;139:1691-700. [PubMed]
- Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. [PubMed]
- Sun PL, Seol H, Lee HJ, et al. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol 2012;7:323-30. [PubMed]
- Rekhtman N, Ang DC, Riely GJ, et al. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol 2013;26:1307-19. [PubMed]
- Russell PA, Barnett SA, Walkiewicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013;8:461-8. [PubMed]
- Lee HJ, Kim YT, Kang CH, et al. Epidermal growth factor receptor mutation in lung adenocarcinomas: relationship with CT characteristics and histologic subtypes. Radiology 2013;268:254-64. [PubMed]
- Shim HS. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 2011;135:1329-34. [PubMed]
- Ninomiya H, Hiramatsu M, Inamura K, et al. Correlation between morphology and EGFR mutations in lung adenocarcinomas Significance of the micropapillary pattern and the hobnail cell type. Lung Cancer 2009;63:235-40. [PubMed]
- Li H, Pan Y, Li Y, et al. Frequency of well-identified oncogenic driver mutations in lung adenocarcinoma of smokers varies with histological subtypes and graduated smoking dose. Lung Cancer 2013;79:8-13. [PubMed]
- Song Z, Zhu H, Guo Z, et al. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Medical oncology 2013;30:645. [PubMed]
- Sumiyoshi S, Yoshizawa A, Sonobe M, et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer 2013;81:53-9. [PubMed]
- Ahn MJ, Lee J, Park YH, et al. Korean ethnicity as compared with white ethnicity is an independent favorable prognostic factor for overall survival in non-small cell lung cancer before and after the oral epidermal growth factor receptor tyrosine kinase inhibitor era. J Thorac Oncol 2010;5:1185-96. [PubMed]
- Kawaguchi T, Matsumura A, Fukai S, et al. Japanese ethnicity compared with Caucasian ethnicity and never-smoking status are independent favorable prognostic factors for overall survival in non-small cell lung cancer: a collaborative epidemiologic study of the National Hospital Organization Study Group for Lung Cancer (NHSGLC) in Japan and a Southern California Regional Cancer Registry databases. J Thorac Oncol 2010;5:1001-10. [PubMed]
- Zhang Y, Sun Y, Shen L, et al. Predictive factors of lymph node status in small peripheral non-small cell lung cancers: tumor histology is more reliable. Ann Surg Oncol 2013;20:1949-54. [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs. lobectomy for lung adenocarcinoma ≤ 2 cm. J Natl Cancer Inst 2013;105:1212-20. [PubMed]
- Rudomina DE, Lin O, Moreira AL. Cytologic diagnosis of pulmonary adenocarcinoma with micropapillary pattern: does it correlate with the histologic findings? Diagn Cytopathol 2009;37:333-9. [PubMed]
- Rodriguez EF, Monaco SE, Dacic S. Cytologic subtyping of lung adenocarcinoma by using the proposed International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) adenocarcinoma classification. Cancer Cytopathol 2013;121:629-37. [PubMed]
- Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol 2012;25:1117-27. [PubMed]
- Kadota K, Yeh Y, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol 2014;27:690-700. [PubMed]
- Kadota K, Nitadori J, Sarkaria IS, et al. Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer 2013;119:931-8. [PubMed]
- Suzuki K, Kadota K, Sima CS, et al. Clinical Impact of Immune Microenvironment in Stage I Lung Adenocarcinoma: Tumor Interleukin-12 Receptor beta2 (IL-12Rbeta2), IL-7R, and Stromal FoxP3/CD3 Ratio Are Independent Predictors of Recurrence. J Clin Oncol 2013;31:490-8. [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [PubMed]
- Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949-55. [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53. [PubMed]
- van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: Selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007;120:868-74. [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [PubMed]
- Girard N, Ostrovnaya I, Lau C, et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res 2009;15:5184-90. [PubMed]
- Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205. [PubMed]
- Sigel CS, Rudomina DE, Sima CS, et al. Predicting pulmonary adenocarcinoma outcome based on a cytology grading system. Cancer Cytopathol 2012;120:35-43. [PubMed]


