Lesion with morphologic feature of organizing pneumonia (OP) in CT-guided lung biopsy samples for diagnosis of bronchiolitis obliterans organizing pneumonia (BOOP): a retrospective study of 134 cases in a single center
Introduction
Bronchiolitis obliterans organizing pneumonia (BOOP) occurs when bronchiolar inflammation rapidly progresses and disrupts areas of the airways due to organizing pneumonia (OP), characterized by loose plugs of granulation tissue (Masson bodies) within bronchioles or alveoli (1-3). Since BOOP was first described by Epler et al. (4) in 1985, it has been reported worldwide as a distinct clinicopathological entity (2). In modern literature, BOOP is distinguished from bronchiolitis obliterans by assignment of the terms cryptogenic (COP) or secondary organizing pneumonia (SOP), based on clinical presentation guidelines (2,5). The term BOOP is used here, as no clear proof exists that COP and SOP represent two distinct clinical entities (6-8).
Since over 80% of BOOP cases are curable (1), accurately distinguishing BOOP from other interstitial lung diseases and pulmonary infectious diseases is critical. However, the BOOP diagnosis as other interstitial pneumonia requires the clinic-radiologic-pathologic data (2) for the typical symptoms and radiographic features are nonspecific, and the pathological hallmark of OP can occur in a variety of other infectious diseases, lung cancer, vasculitis and so on (3,9). Since the OP is nonspecific, a rather large piece of lung tissue is required (2,5). The surgical lung biopsy is recommended by 2002 ATS/ERS Consensus Classification of the Idiopathic Interstitial Pneumonias for the diagnostic accuracy is about 90% (2,5,9-14). Such specimens, however, require general anesthesia and cases incur substantial morbidity and mortality risks (13-15). Safer and more effective methods for identifying BOOP-associated OP are urgently needed.
Though transbronchial lung biopsy (TBLB) is an important minimally invasive lung biopsy method, only 7-37% of patients are revealed in these samples (5,10). Thus, TBLB may be of limited use in BOOP diagnosis (5,10). Alternatively, several recent studies (16-18) have applied CT-guided lung biopsy for diagnosis of lung lesions, producing acceptable results with relatively lower costs and complication rates. CT-guided lung biopsy for BOOP has been reported in isolated cases (19-21); however, no broad clinical assessments of accuracy in small biopsy specimens with non-specific OP have been conducted (5).
To investigate the effectiveness of diagnosing BOOP using specimens produced by CT-guided lung biopsy in patients with non-specific OP, a retrospective analysis was conducted. These findings allow for the evaluation of OP in CT-guided lung biopsy specimens, as a reliably pathological evidence for BOOP.
Materials and methods
The study protocol was approved by the Drum Tower Hospital Institutional Review Board of the Medical School of Nanjing University (Nanjing, China) prior to beginning the study. Patient consent was not required for the retrospective study. The diagnostic pathology archives of the Drum Tower Hospital were searched for cases in which the terms ‘‘OP’’ and “CT-guided lung biopsy” were present. A total of 134 cases from 1 January 2004 to 31 December 2011 were identified. The morphologic feature of OP using published histological criteria (2,3,5) was confirmed by two independent and experienced pathologists. Eleven patients were excluded from the 134 cases due to the OP was not the only finding in the tissue: vasculitis (three cases), suspected pulmonary abscess (one patient), pulmonary tuberculosis (one patient), cryptococcus pneumonia (two cases), pulmonary aspergillosis (two cases), pulmonary infarction (one patient), and low-grade pulmonary lymphomas (one patient).
Then clinical data were collected by computerized searching in the Patient Record database and image database. The data were made up by the demographic data; symptoms and signs; routine laboratory tests including full blood cell counts, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP); re-biopsy findings from surgery, TBLB, or CT-guided lung biopsy; prescribed medications and images (or reports) of plain chest radiographs and/or CT findings. A minimum 3 months follow-up record was needed for the improvement either by spontaneous, surgery or treatment.
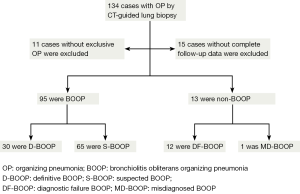
Of the 123 patients, 15 patients were excluded due to an absence of the minimum follow-up of 3 months for review. Accordingly, 108 cases with exclusive OP finding, complete patients’ notes, radiographic imagines or reports, and follow-up data made up the final study group. The patients data were reviewed by five pulmonologists using published criteria of BOOP (2,22). In brief: (I) radiographic abnormalities ranging from multiple acinar/nodular shadows to solitary pneumonia-like or nodular shadows; (II) exclusive OP findings-masson bodies were apparent in alveoli and chronic inflammatory cell infiltration in the mesenchyme (Figure 1)—in the CT-guided lung biopsy samples; (III) negative microbiological analysis or no response to standard antibiotic therapy; (IV) rapid clinical and imaging improvement following corticosteroid treatment (or spontaneous in cases not prescribe these medications). Cases consistent with BOOP were assigned for BOOP group, or they were assigned for non-BOOP group.
To evaluate the role of the nonspecific OP findings by CT-guided lung biopsy, BOOP patients were assigned for definitive BOOP (D-BOOP) and suspected BOOP (S-BOOP) group when the patients were initially diagnosed as probable, possible BOOP according to the clinical and radiographic data alone, respectively. Non-BOOP patients were assigned for diagnostic failure BOOP (DF-BOOP) and misdiagnosed BOOP (MD-BOOP) group. DF-BOOP patients were initially diagnosed as other diseases and the exclusive OP by CT-guided lung biopsy confused the diagnosis and need re-biopsy. MD-BOOP patients were misdiagnosed according to the clinical, radiographic, exclusive OP by CT-guided lung biopsy (Figure 2).
Statistical analysis
All statistical analyses were conducted using SPSS version 17.0 for Windows (IBM Inc., Chicago, IL, USA). Patient characteristics were expressed as means ± SD or number and frequency percentages. Group differences were tested by t-tests or χ2 tests. P values less than 0.05 were considered significant (P<0.05).
Results
Clinical data
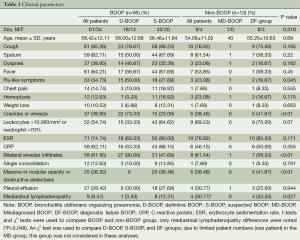
Clinical data are summarized in Table 1. A total of 108 patients finally fulfilled the inclusion criteria, with follow-up times ranging from 3-51 months (mean 9.86±6.23 months) with intervals of 1-12 months (mean 3.45±1.81 months). Of the108 cases, 95 (87.97%) cases and 13 (12.03%) cases were classified as BOOP and non-BOOP group, respectively. In the BOOP group, 61 (64.21%) were male and the mean age was 58.42±12.11 years (range, 22-77 years). Presenting symptoms and signs included cough, sputum, dyspnea, fever, flu-like symptoms, chest pain, hemoptysis, weight loss and crackle or wheeze and the laboratory data are no significant differences between the BOOP and non-BOOP group (P>0.05). The difference of the imaging features between the two groups was the mediastinal lymphadenopathy (P=0.048).
Full table
The BOOP group included 30 (31.58%) D-BOOP and 65 (68.42%) S-BOOP cases. The non-BOOP group included 1 (7.70%) MD-BOOP and 12 (92.30%) DF-group cases (Figure 2). As there was only one patient in the MD-BOOP group, no comparison with other groups was performed.
Observations in D-BOOP group
D-BOOP patients who were firstly diagnosed as probable BOOP only according to the clinical and radiographic data were supported by the OP feature in the CT-guided lung biopsy samples and verified by the rapid improvement after treatment. It seems that those patients’ data including symptoms and signs and imaging features are more typical than other groups. It was well known that BOOP begin with a mild flu-like illness with fever, cough, malaise, dyspnea, weight loss and others (2,5,9). In this study, flu-like illnesses, fever, cough and crackles were presented more than 50% of D-BOOP cases. Only flu-like symptoms and crackles were significantly more common in the D-BOOP group than in the S-BOOP or DF-BOOP groups (P<0.05). Other manifestations such as mild dyspnea, anorexia, and weight loss were no significant differences between them (Table 1).
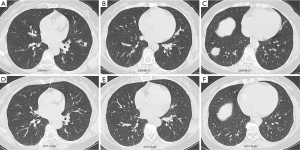
Twenty-seven (90%) of D-BOOP cases exhibited multiple alveolar filling shadows in both lungs with densities ranging from ground-glass to consolidation ranging several centimeters to a full lobe (Figure 3). Most were peripheral and some exhibited migratory patterns. Air bronchograms were visible among consolidation. Notably, these were assigned for the typical BOOP imaging features and less in either the S-BOOP group or DF-group in this study (47.7% and 58.3%, respectively; P<0.01). Additionally, three cases exhibited unilateral opacities firstly diagnosed as BOOP based on clinical manifestations along (no obvious fever; normal WBC count, ESR, and CRP level; present cough, dyspnea, and auscultation crackles).
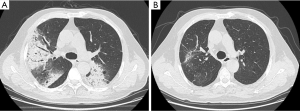
With corticosteroid therapy, D-BOOP cases, including relapsed cases, exhibited overall positive treatment outcomes. A total of 30 D-BOOP cases exhibited lesions with rapid resolution following glucocorticoid treatment. There were no residues in 23 cases, and only 7 cases exhibited apparent traction bronchiectasis or bands of fibrous tissue (Figure 3).
Observations in S-BOOP group
S-BOOP patients who were as possible BOOP based on the clinical and radiographic data alone were confirmed by the pathological hallmark in CT-guided lung biopsy specimens and reinforced by the rapid improvement with corticosteroid therapy.
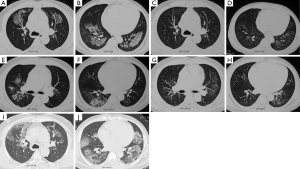
Of the 65 cases, 25 (38.46%) cases were suspected as tumor for radiographic features with single or multiple nodules (Figure 4) and obstructive pneumonia-like structural abnormalities. Forty cases (61.54%) were initially diagnosed as bacterial or fungal infections or tuberculosis that did not respond to antibiotic treatment. Fortunately, 51 patients were established the diagnosis of BOOP according to the OP findings in the CT-guided lung biopsy tissues and avoid the surgical lung biopsy. In view of small biopsy sample limitations and non-specific OP change, 14 cases with atypical imaging findings were required the re-biopsy, either with bronchoscope, secondary CT-guided lung biopsy, or surgical lesion excision.
The S-BOOP group as in the D-BOOP group, lesions rapidly regressed in most cases with corticosteroid treatment (64 cases), with minimal bands or streaks remaining in only 19 cases. One patient with OP by CT-guided lung biopsy was initially suspected as lung cancer by CT scanning. Though this patient voluntarily rejected further diagnosis and treatment, the lesion resolved spontaneously within 32 months, confirming a correct diagnosis of BOOP rather than cancer (Figure 4).
Observations in MD-BOOP group
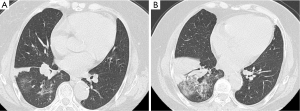
One patient, a 40-year-old male, was misdiagnosed with BOOP based on symptoms of dry cough and shortness of breath after activity, and radiography of multiple alveolar infiltrates and OP by biopsy. Rapid clinical and imaging improvement at rest was improperly regarded as the effect of corticosteroid treatment at the first 3 months period (Figure 5). When migratory lesions appeared, the final confirmed diagnosis of cardiac tumor was established.
Observations in DF-group
Of the 12 cases, no clear clinical manifestations implied BOOP. Radiography revealed suspect masses in five cases, and seven cases exhibited multiple opacities accompanied by cavity-like changes. Pleural effusion was observed in three cases, two cases exhibited moderate pleural effusion. Four cases exhibited enlarged lymph nodes, and four cases were diagnosed with other diseases, including vasculitis, occult nephritis, leukemia, and diabetes. Moreover, one patient exhibited eosinophil levels >30%.
The OP by CT-guided lung biopsy confused the diagnosis. As in the S-BOOP group, further confirmation by biopsy was required for ten cases. A total of 12 DF-group cases were finally diagnosed with pneumonia (four cases), tuberculosis (three cases), and pulmonary fungal infections (two cases), lung cancer (one patient) (Figure 6), lung abscess (one patient), and eosinophilic pneumonia (one case). All cases exhibited good recovery with appropriate treatment.
OP by CT-guided lung biopsy versus clinical and radiologic data alone for BOOP diagnosis
Based on clinical and radiologic data alone, BOOP was only diagnosed correctly in 30 (31.25%) out of 96 cases (BOOP and MD-BOOP) and was excluded in 12 (11.11%, DF-BOOP) out of 108 cases. Application of the exclusive OP by CT-guided lung biopsy alone, the diagnostic accuracy of BOOP was 87.96% (95/108). As the recommended diagnostic process of 2002 ATS/ERS Consensus Classification of the Idiopathic Interstitial Pneumonias (2), combined clinical, radiographic, and pathological findings- the exclusive OP by CT-guided lung biopsy instead of surgical lung biopsy-produced an amazing diagnostic accuracy of 98.96% (95/96).
Discussion
The current study demonstrated that the exclusive OP finding by CT-guided lung biopsy could be used as a reliably pathological evidence for BOOP diagnosis, especially combined with clinical and radiographic data could further increase the diagnostic accuracy.
The diagnostic process of the interstitial pneumonias is an integrated clinic-radiologic-pathologic approach (2). The clinical manifestations including symptoms and signs and imaging features between BOOP and non-BOOP are not highly differences; however, they are the good preliminary indicators. As in the D-BOOP group and DF-BOOP, the typical imaging features could distinguish BOOP from non-BOOP at initial diagnostic process. Notably, the typical BOOP imaging features and crackle are the confident evidence for pulmonologists, but the diagnostic accuracy in the present study was 31.25% (D-BOOP), more patients were often compounding initial diagnoses and needed further evidence to establish the diagnosis (S-BOOP). Though significantly higher reports have been published, indicating up to 79% diagnostic accuracy (23), these discrepancies may be due to better identification of BOOP in idiopathic interstitial pneumonia using CT imaging features.
Widely accepted surgical lung biopsy is a costly procedure that may unnecessarily increase the risk of serious complications in some cases. Alternatively, Jara-Palomares et al. (22) reported that high resolution CT findings and bronchoalveolar lavage could be used in cases with suspicious clinical presentations. However, no extensive clinical trials have assessed these techniques in BOOP cases. OP by CT-guided lung biopsy produced a current diagnostic accuracy for BOOP of 87.96%, much higher than that of TBLB reported to range from 7% to 37% (10). Especially in cases with non-specific clinical and radiological features as in the S-BOOP group, commonly misdiagnosed as lung cancer, risky and unnecessary surgery is often performed (24). The current findings clearly demonstrate that OP by CT-guided lung biopsy can obviate the need for surgery lung biopsy, although some cases still required additional OP using more invasive techniques. Thus, OP by CT-guided lung biopsy is an acceptable result for BOOP diagnosis that may increase the accuracy of BOOP patient diagnosis and facilitate early intervention. In view of CT-guided lung biopsy is much more affordably and safer method than conventional invasive surgical biopsy in the previous reports (2,10-15,17,18) and has been proved the feasibility in early diagnosis of BOOP in some cases reports (19,20), our findings suggested that CT-guided lung biopsy could be as a reasonable alternative to lung biopsy for practically effective early diagnosis of BOOP.
Notably, identification of OP is the predominant diagnostic criteria for BOOP; however, OP changes were not always related to BOOP as previously believed (2,3,5,25) and in current study (11 patients were excluded from the 134 cases due to the OP was not the only finding in the tissue and 12 patients with OP in the DF-BOOP were finally diagnosed the other diseases). Because of the misdiagnosing alternative conditions that may mimic BOOP (3,5), our observations highlight some key concepts regarding integration of radiographic characteristics, symptomatic manifestations, OP by CT-guided lung biopsy, proper treatment, and observation to avoid the misdiagnosis. In current study, one patient in MD-BOOP with carefully follow-up was finally corrected diagnosis as cardiac tumors. The combined diagnostic accuracy of BOOP reaches 98.96% (95/96).
Additionally, not all BOOP cases presenting typical imaging features, such as bronchocentric patterns and reversed halo signs (3,26-28), were considered in the present study due to ineligibility of for CT-guided lung biopsy, potentially limiting the applicability of these results to broader patient populations. Further, limitations of this study are its retrospective nature, but we believe that the largest in literature to date, confirms the OP by CT-guided lung biopsy as a reliable evidence for BOOP.
Conclusions
The current retrospective analysis indicated that the diagnostic accuracy of OP by CT-guided lung biopsy for BOOP was 87.96%, a value slightly lower than previously reported using surgical lung biopsy techniques but higher than that reported using TBLB. Thus the OP by CT-guided lung biopsy can aid in early detection of BOOP, particularly when combined with clinical and radiological data. In view of CT-guided lung biopsy is affordably and safe method, it can be used to confirm OP pathology reliably and reduce unnecessary surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Epler GR. Bronchiolitis obliterans organizing pneumonia, 25 years: a variety of causes, but what are the treatment options? Expert Rev Respir Med 2011;5:353-61. [PubMed]
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Roberton BJ, Hansell DM. Organizing pneumonia: a kaleidoscope of concepts and morphologies. Eur Radiol 2011;21:2244-54. [PubMed]
- Epler GR, Colby TV, McLoud TC, et al. Bronchiolitis obliterans organizing pneumonia. N Engl J Med 1985;312:152-8. [PubMed]
- Cottin V, Cordier JF. Cryptogenic organizing pneumonia. Semin Respir Crit Care Med 2012;33:462-75. [PubMed]
- Basarakodu KR, Aronow WS, Nair CK, et al. Differences in treatment and in outcomes between idiopathic and secondary forms of organizing pneumonia. Am J Ther 2007;14:422-6. [PubMed]
- Vasu TS, Cavallazzi R, Hirani A, et al. Clinical and radiologic distinctions between secondary bronchiolitis obliterans organizing pneumonia and cryptogenic organizing pneumonia. Respir Care 2009;54:1028-32. [PubMed]
- Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, et al. Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response, and prognosis. Chest 2011;139:893-900. [PubMed]
- Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006;28:422-46. [PubMed]
- King TE Jr. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 2005;172:268-79. [PubMed]
- Zhang D, Liu Y. Surgical lung biopsies in 418 patients with suspected interstitial lung disease in China. Intern Med 2010;49:1097-102. [PubMed]
- Carnochan FM, Walker WS, Cameron EW. Efficacy of video assisted thoracoscopic lung biopsy: an historical comparison with open lung biopsy. Thorax 1994;49:361-3. [PubMed]
- Sigurdsson MI, Isaksson HJ, Gudmundsson G, et al. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: a retrospective study. Ann Thorac Surg 2009;88:227-32. [PubMed]
- Poletti V, Chilosi M, Olivieri D. Diagnostic invasive procedures in diffuse infiltrative lung diseases. Respiration 2004;71:107-19. [PubMed]
- Riley DJ. Risk of surgical lung biopsy in idiopathic interstitial pneumonias. Chest 2005;127:1485-6. [PubMed]
- Doxtader EE, Mukhopadhyay S, Katzenstein AL. Core needle biopsy in benign lung lesions: pathologic findings in 159 cases. Hum Pathol 2010;41:1530-5. [PubMed]
- Yuan DM, Lü YL, Yao YW, et al. Diagnostic efficiency and complication rate of CT-guided lung biopsy: a single center experience of the procedures conducted over a 10-year period. Chin Med J (Engl) 2011;124:3227-31. [PubMed]
- Anderson JM, Murchison J, Patel D. CT-guided lung biopsy: factors influencing diagnostic yield and complication rate. Clin Radiol 2003;58:791-7. [PubMed]
- Yebra M, Romero Y, Varela A, et al. Percutaneous lung biopsy in the diagnosis of bronchiolitis obliterans organizing pneumonia. Chest 1994;105:972-3. [PubMed]
- Poulou LS, Tsangaridou I, Filippoussis P, et al. Feasibility of CT-guided percutaneous needle biopsy in early diagnosis of BOOP. Cardiovasc Intervent Radiol 2008;31:1003-7. [PubMed]
- Metzger F, Pernet D, Manzoni P, et al. The contribution of CT-guided transthoracic lung biopsy to the diagnosis of organising pneumonia. Rev Mal Respir 2010;27:e6-16. [PubMed]
- Jara-Palomares L, Gomez-Izquierdo L, Gonzalez-Vergara D, et al. Utility of high-resolution computed tomography and BAL in cryptogenic organizing pneumonia. Respir Med 2010;104:1706-11. [PubMed]
- Johkoh T, Müller NL, Cartier Y, et al. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology 1999;211:555-60. [PubMed]
- Zheng Z, Pan Y, Song C, et al. Focal organizing pneumonia mimicking lung cancer: a surgeon's view. Am Surg 2012;78:133-7. [PubMed]
- White KA, Ruth-Sahd LA. Bronchiolitis obliterans organizing pneumonia. Crit Care Nurse 2007;27:53-66. [PubMed]
- Lee JW, Lee KS, Lee HY, et al. Cryptogenic organizing pneumonia: serial high-resolution CT findings in 22 patients. AJR Am J Roentgenol 2010;195:916-22. [PubMed]
- Arakawa H, Kurihara Y, Niimi H, et al. Bronchiolitis obliterans with organizing pneumonia versus chronic eosinophilic pneumonia: high-resolution CT findings in 81 patients. AJR Am J Roentgenol 2001;176:1053-8. [PubMed]
- Kim SJ, Lee KS, Ryu YH, et al. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol 2003;180:1251-4. [PubMed]