Ex vivo lung perfusion
The shortage of donor lungs
According to the Thirtieth Adult Lung and Heart-Lung Transplant Report 2013, from the Registry of the International Society for Heart and Lung Transplantation, lung transplantation (LTx) is a therapy that is being performed worldwide, with numbers increasing every year (1). In 2011, 3,640 LTxs were reported compared to only 1,712 annual cases a decade ago. As the outcomes tend to improve, an increasing number of patients with end-stage lung disease are being considered for LTx. Nevertheless, the amount of lungs suitable for transplantation has not followed this trend and this equation generates considerable waitlist mortality (15.4 per 100 wait-list years in the US form 2010 to 2012) (2).
Donor lungs are subjected to several injurious mechanisms during the brain death/organ donation process (such as ventilator-acquired pneumonia, neurogenic and hydrostatic pulmonary edema, barotrauma). Thus, it is not surprising that the majority of donor lungs are not utilized for transplantation (39% Eurotransplant 2012, 78% SRTR in the US 2012).
Strategies for lung donor pool expansion
Expansion of the donor pool has been attempted by extending the donor selection conventional criteria, by use of donation after cardiac death (DCD) and, lastly, with the implementation of ex vivo lung perfusion (EVLP). The ideal donor corresponds to a <55 year-old with <20 pack-year smoking history, no chest trauma, clear chest X-ray, P/F >300 and absence of purulent secretions and organisms on gram stain of respiratory samples. This scenario is known to correspond to less than half of the donors utilized for transplantation (3). Several studies addressing the use of extended criteria donors have been published and, more recently, a review study summarized the findings of 10 studies ranging from 1993 to 2010, bringing the best evidence up to date (4). Although no clear differences in mid or long-term survival were observed, 4 of these studies revealed worse early outcomes (such as 30- and 90-day mortality, ICU and hospital stay and gas exchange at ICU arrival). Recently, the Hannover group has shown an interesting algorithm proposing allocation of extended criteria donor lungs to lower-risk recipients. Results were encouraging and deserve further analysis (5).
Although the first successful LTx was performed from DCD, the concept of using controlled DCD lungs has been clinically revisited by D’Alessandro et al., in 1995 (6). Series of studies have followed, reporting an increasing international experience and highlighting the potential of DCD to partially address the shortage of donor lungs (7-13). Nevertheless, caution is still observed in the transplant community as there are a series of specific injuries that the DCD lung is prone to, specially during the interval from withdrawal of life sustaining therapies to pulmonary artery (PA) flush. Another potential source of lungs comes from the use of uncontrolled DCDs (Maastricht categories I and II). The group of Madrid has explored this peculiar pool, reporting the experience with 29 cases. Ninety-day and 1-year mortality were 22% and 32% respectively, with higher rates of primary graft dysfunction (PGD) 2-3 than expected (14).
The use of lungs from smoker donors has been recently studied in a large registry database including 1,295 transplants (510 with smoking history) from UK. Despite presenting worse 3-year survival, the use of lungs from donors with a positive smoking history was shown to provide a survival benefit for patients with interstitial lung disease listed for transplantation (15). Several recent studies followed and supported the use of such donors (16-18). Nevertheless, caution was raised in the analysis of the UNOS database including 3,704 single-lung transplants from 2005 to 2011. In this modality of transplant, recipients from donors with an active smoking history, but not those from donors that quit smoking, were associated with increased mortality (19).
Lastly, clinical EVLP was shown to safely increase the donor pool by preserving high-risk donor lungs with similar outcomes to standard criteria donor lungs (20). This review will focus on technical aspects of EVLP, its recent clinical experience and pre-clinical application in DCD.
EX vivo lung perfusion
Perfusion of whole organs was initially envisioned by Alexis Carrel and Charles Lindbergh. In the 30’s, they performed several experiments with organs such as heart, kidney, thyroid, ovary, adrenal glands and spleen (21). Up to the 90’s, experiments with lung perfusion were viewed as a reliable method to study pulmonary physiology. The first clinical application was described by Steen and coworkers at University Hospital of Lund. In 2001, they described the utilization of EVLP to assess the lungs of a 54-year-old who suffered a myocardial infarction while admitted to the intensive care unit. Lungs were topically cooled with perfadex and procured after 190 minutes of cardiopulmonary resuscitation cessation. EVLP was performed for 65 minutes and a successful right single lung transplant was performed (22). The same group further expanded the application of short-period EVLP to lungs initially rejected for transplantation. A total of 6 sets of donor lungs were perfused from 61 to 121 minutes, rendering six successful double lung transplants (23). The Toronto group mastered the technique and introduced the concept of extended EVLP, focusing not only on reassessment but also on providing a platform for treatment delivery in the normothermic state (24,25).
EVLP—the Toronto technique
The foundations of our current technique for clinical EVLP are: (I) gradual rewarming up to normothermia; (II) gradual increase in vascular flow as the lungs are rewarmed, targeting 40% of the donor predicted cardiac output; (III) protective lung ventilation; (IV) acellular perfusate with increased colloid osmotic pressure.
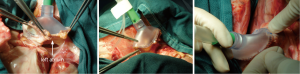
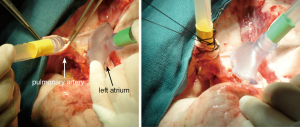
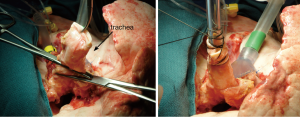
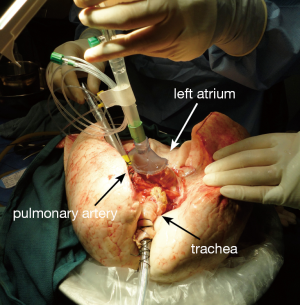
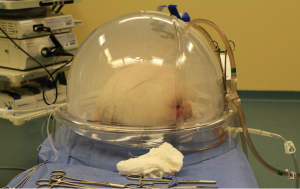
The indications for EVLP are listed in Table 1. Once at the transplant center, the lungs are dissected on the back table. The left atrial (LA) cuff is trimmed and sewn to a dedicated cannula with two 4-0 polypropylene running sutures (Figure 1). If adequate length on the PA is available, the PA cannula can be simply inserted proximal to its bifurcation and secured with two heavy silk ties (Figure 2). In cases of short main PA—usually in concomitant heart procurement—a cuffed PA cannula can be sewn with two 5-0 polypropylene running sutures, similarly to the atrium. With the trachea clamped at the level of the carina, the staple line is opened and a conventional endotracheal tube is inserted and secured with two heavy silk ties (Figure 3). A second retrograde flush with 1L of Perfadex is performed. The inflated lungs are then taken to the EVLP dome and ready to be connected to the circuit (Figure 4). If one of the lungs is judged too damaged for clinical EVLP (e.g., due to pneumonia), the contralateral lung can be perfused alone. Care should be taken to keep adequate arterial and atrial cuffs and a long trachea/bronchus at the moment of division.

Full table
The EVLP circuit
A dedicated circuit composed of a centrifugal pump, a leukocyte filter, a hollow-fiber oxygenator heat exchanger and a hardshell reservoir is currently used. It is primed with 2.0 L of Steen solution (XVIVO, Vitrolife), 500 mg methylprednisolone (Solu-medrol; Sandoz Canada, Boucherville, Canada), 3,000 IU of unfractionated heparin (Organon, Canada) and antibiotic (500 mg imipenem/cilastatin, Primaxin; Merck, Whitehouse Station, NJ).
Initiation and steady state
Once on the EVLP dome, a cotton sponge is positioned beneath the lung block to prevent excessive sliding. Antegrade flow is commenced through the PA cannula, which is attached to the circuit once appropriate deairing is achieved. The LA cannula is then deaired and connected to the circuit. The outflow clamp is now removed. Our target perfusion flow consists of 40% of the donor predicted cardiac output. Following our principles of gradual rewarming and stepwise increase in vascular flow, the procedure is then initiated with lungs on room temperature and perfusion with 10% of the calculated target flow. At 10 minutes, the flow is raised to 20% of predicted and the temperature is set to 30 °C. At subsequent 10-minute time points (20, 30, 40 and 50 minutes), the flow is increased to 30%, 50%, 80% and finally 100% of target, respectively. Furthermore, the temperature is set to 37 °C at 20 minutes and ventilation is initiated (7 mL/kg, PEEP 5 cm H2O and 7 cycles/min) when the temperature reaches 33 °C. Once the lungs are being ventilated, the gas mixture (86% N2, 8% CO2 and 6% O2) is turned on at a sweep of 1 L/min. The target of a post-membrane pCO2 between 35-40 mmHg is achieved by titrating the sweep gas. Lastly, the left atrial pressure should be carefully maintained in the 3-5 mmHg range by adjusting the level of the reservoir. Once the lungs are normothermic, ventilated and target flow is achieved, recruitment maneuvers are performed up to 25 cm H2O. The lungs have now reached the steady state (Figure 5). Steen solution is exchanged from the circuit hourly, 500 mL in the first hour followed by 250 mL thereafter.
Assessment mode
Assessment is performed hourly. Ventilation parameters are set to 10 mL/kg tidal volume, 10 breaths per minute and FiO2 1.0 for five minutes. PA pressure, LA pressure, peak airway pressure, plateau pressure, dynamic and static compliance are recorded. Perfusate gas analysis is done in samples taken from the venous and arterial sides. At 1 hour of EVLP and then every two hours, a lung X-ray is routinely performed. Criteria for lung acceptance or declination for transplantation after EVLP are displayed in Table 2. One should notice that the acellular nature of the Steen solution makes perfusate pO2 a later marker of lung injury. As demonstrated by Yeung and coworkers, compliance and peak airway pressure deterioration are observed before changes in perfusate pO2 (27). Pulmonary recruitment is performed every 30 minutes after each assessment by increasing the tidal volume with subsequent inspiratory hold maneuvers up to 25 cm H2O for ten seconds.
Termination of perfusion
Our clinical protocol includes EVLP for four to six hours. Frequently, it is possible to make the decision at three hours (3 assessments, 2 lung X-rays) and send for the recipient. By the fourth hour the recipient will be relatively ready for skin incision. Nevertheless, if no clear decision can be made at this time point, perfusion can be extended for up to 6 hours.
Once decision is made to terminate perfusion, lungs are ventilated with 0.5 FiO2 and cooled to 15 °C. The inflow and outflow cannulae are clamped and cut. The endotracheal tube is clamped as well with special attention to maintain the lungs inflated. A last antegrade flush is performed with 500 mL of Steen solution. The vascular cannulae are removed and the trachea is stapled just below the endotracheal tube. Topical cooling with Perfadex and ice follows the same steps of conventional preservation and the lungs are taken to the recipient OR inside a cooler.
Worldwide experience with clinical EVLP
The Toronto technique
The Toronto Lung Transplant Program conducted a nonrandomized clinical trial to assess the feasibility of EVLP selecting high-risk donor lungs for this modality of preservation (20). A total of 23 donor lungs were submitted to EVLP with 20 being ultimately transplanted (15 bilateral and 5 unilateral lung transplants). The primary end-point of the study (PGD grade 2 or 3 at 72 hours) was recorded in 15% of the EVLP group and 30% of the contemporary no EVLP controls (116 cases), with no significant difference. Secondary end-points such as PGD 2 or 3 at ICU arrival, 24 and 48 hours; ECLS requirement; days on mechanical ventilation; ICU stay; hospital stay and 30-day mortality were also comparable between groups. This experience was recently updated with a total of 50 lung transplants from 58 EVLPs (86% yield) (26). In the study period, from September 2008 to December 2011, 253 lung transplants were performed with conventional preservation lungs. PGD 3 at 72 hours was recorded in 2% EVLP vs. 8.5% control (P=0.14). Again, time on mechanical ventilation, ECLS requirement, ICU stay, hospital stay and 30-day mortality were not different. Furthermore, similar 1-year survivals were observed: 87% for EVLP group vs. 86% for the standard group.
In 2012, the group from Vienna reported their experience with 13 clinical EVLPs which rendered nine double-lung transplants (69% yield) (28). Early outcomes such as days on mechanical ventilation, ICU stay, hospital stay and 30-day mortality were comparable to 119 contemporary conventional preservation transplants. Of notice, some modifications from the Toronto technique were implemented: (I) decision was made at two hours of perfusion if physiologic parameters were met; (II) recruitment maneuvers were performed 10 minutes before assessments (as opposed to 30 minutes); (III) lungs were ventilated for 15 minutes on 1.0 FiO2 for each assessment (as opposed to five minutes). Interestingly, all the four declined cases developed massive pulmonary edema and were recovered from donors with trauma history.
The groups from Toronto, Vienna and Paris presented their clinical EVLP experience at the 2013 ISHLT meeting (29). A total of 125 clinical EVLPs were performed with an 82.5% yield. Similarly to previous uni-institutional reports, the incidence of PGD3 at 72 hours was 5% and the 12-month mortality was 12%.
In 2012, the Harefield Hospital (UK) reported six double lung transplants generated from 13 EVLPs (yield 46%) (30). Although the median requirement of mechanical ventilation post-transplant was greater than seven days, all patients ultimately left the hospital and were alive at three months. The Toronto technique was implemented with some modifications, such as shorter perfusion times (average 2 hours) and no interval lung X-ray in 50% of the accepted cases.
The group of Torino described nine EVLPs rendering seven lung transplants (yield 78%) (31). These cases corresponded to 30% of their LTx activity and illustrated the impact of EVLP on lower volume centers.
In the Newcastle experience with 6 lung transplants from 18 EVLPs (yield 33%), all patients survived to hospital discharge (32). Furthermore, this report pointed to a possible benefit of EVLP: bacterial loads in bronchoalveolar lavages at the end of EVLP were significantly lower than on samples taken at its initiation. Authors also reported that, despite decrease in the bacterial loads there was an increase in the load of Candida sp. in two of their first three cases. After this observation, Amphotericin B was routinely added to the perfusate. Further studies are required to better elucidate the role of anti-fungal therapy in EVLP.
The NOVEL Lung trial is an FDA mandated multicenter clinical trial (NOVEL Lung Trial) studying EVLP for marginal donors. The initial report included 31 patients that received EVLP lungs. Early outcomes such as PGD, length on mechanical ventilation, ICU stay, hospital stay and 30-day mortality were similar to 31 non-EVLP controls (33). At the 2014 ISHLT meeting, the trial results were updated to 76 EVLPs rendering 42 lung transplants (55% conversion rate) (34). In comparison with 42 contemporary controls, early outcomes and 1-year survival were not different.
The Lund technique
The main differences from the Toronto technique reside in the open left atrium, the use of Steen solution mixed with red blood cells and the perfusion at flows correspondent to 100% of the donor predicted cardiac output (35).
Following the successful case in 2001 (22), Steen and coworkers reported the use of EVLP for the evaluation of 9 donors lungs rejected for transplantation (23). Ultimately, 6 double lung transplants were performed, representing 35% of the lung transplant activity for the study period. Although two patients died early on the post-transplant course (one 63-year-old COPD male died at 95 days due to sepsis and multi-organ dysfunction; one 64-year-old COPD female died at 9 months due to rejection); the remaining four were followed for almost 2 years and presented good lung function.
The group from University of Gothenburg has reported their outcomes with 11 EVLPs over an 18-month period (36). A total of eight double and three single LTxs were performed and, although hospital stays were similar, the time on mechanical ventilation and ICU length of stay were longer in the EVLP group compared to conventional transplants. Nevertheless, there was no hospital mortality in the EVLP group.
Reflecting the widepread utilization of EVLP by LTx programs throughout the world, the group from Copenhagen recently reported the Danish experience with 7 EVLP lung transplants (37). This number corresponded to 21% of the yearly activity and the outcomes were favorable despite one death at 104 days post-transplant due to Mycobacterium abscessus infection.
The portable ex vivo technique
This system is capable of transportation in addition to ventilation/perfusion. Similarly to the Lund technique, the left atrium is kept open and red blood cells are added to the perfusate (a modified low-potassium dextran solution). The perfusion flow is set to 2.5 L/min (38).
A pilot study assessing preservation and transportation of conventional criteria donor lungs was published in 2012 by the programs of Hannover and Madrid (38). A total of 12 patients were transplanted, with perfusion times ranging from 188 to 622 minutes. All cases were bilateral LTxs and there was no PGD 3 at 72 hours. Currently there is an ongoing multicentre clinical trial assessing the feasibility and potential benefits of this strategy for extended criteria donor lungs.
EVLP as a platform for assessment and treatment of DCD lungs
There is a growing body of research focusing on the application of EVLP to assess and repair DCD lungs. The low clinical utilization rates of these lungs are likely driven by the different injuries (such as warm ischemia, hypoxia, hypotension and aspiration) that they are prone when compared to neurological determination of death donors (39). The potential of EVLP to further refine DCD lung selection is well illustrated by the pre-clinical report of Sanchez et al., showing that improved endothelial function reflected in better EVLP physiological performance in porcine lungs treated with pre-arrest heparinization (40).
Furthermore, the use of EVLP as a platform to deliver different medications has been tested and proved to be beneficial in most reports. Nakajima and coworkers have added nitroglycerin and dibutyryl cyclic adenosine monophosphate to Steen solution during EVLP of lungs submitted to 4 hours of warm ischemia (41). After single LTx, EVLP lungs had better function, lower histological signs of acute lung injury and improved microvascular patency compared to conventional preservation lungs. Mulloy and coworkers (42) added a selective adenosine 2A agonist to the perfusate in a model of one hour of warm ischemia in pigs. After procurement, lungs submitted to extra four hours of cold ischemia and then four hours of EVLP performed significantly better than lungs submitted to four hours of cold ischemia only, with less histological lung injury and lower levels of inflammatory cytokines in the bronchoalveolar lavage after single left LTx.
Lastly, some groups have moved further with the clinical use of uncontrolled DCDs. Pioneer work from the Hospital Universitario Puerta de Hierro has initially shown high incidence of PGD3 (38%), with 17% hospital mortality and 57% 1-year survival from 29 uncontrolled DCD LTxs (43). The addition of EVLP to this algorithm helped to better select this lungs and rendered no case of PGD3 in the initial 4 EVLP LTxs, with additional exclusion of four lungs with poor EVLP performance (44). More recently, Tom Egan has shown the feasibility of a similar approach in a US clinical trial, having procured and perfused two uncontrolled DCD lungs. Although one of them deteriorated on the circuit, the other one presented adequate function and was not transplanted only because there was no recipient to match blood type and size (45).
The future
The current EVLP assessment is mainly based on physiological parameters, added to lung X-ray, bronchoscopy and macroscopic evaluation. Although EVLP has provided similar results of LTx with extended criteria donor lungs compared to those with conventional ones, we still observe a small percentage of PGD3. Certainly one cannot control for recipient factors, nevertheless, the addition of biomarkers to EVLP assessment has the potential to further refine donor lung selection. Since plausible biomarker candidates have been suggested, the next barrier to clinical translation resides in the design of rapid diagnostic assays in order not only to validate but also to provide this information in a timely fashion.
Acknowledgements
Disclosure: M. Cypel was a principal investigator for the Toronto Ex-Vivo Lung Perfusion Trial sponsored by Vitrolife, a company that makes sterile solutions for organ preservation. M. Cypel is a founding member of Perfusix Inc., a company that provides ex-vivo organ perfusion services.
References
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [PubMed]
- Valapour M, Paulson K, Smith JM, et al. OPTN/SRTR 2011 Annual Data Report: lung. Am J Transplant 2013;13 Suppl 1:149-77. [PubMed]
- Pierre AF, Sekine Y, Hutcheon MA, et al. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg. 2002;123:421-7; discussion, 427-8. [PubMed]
- Schiavon M, Falcoz PE, Santelmo N, et al. Does the use of extended criteria donors influence early and long-term results of lung transplantation? Interact Cardiovasc Thorac Surg 2012;14:183-7. [PubMed]
- Sommer W, Kühn C, Tudorache I, et al. Extended criteria donor lungs and clinical outcome: results of an alternative allocation algorithm. J Heart Lung Transplant 2013;32:1065-72. [PubMed]
- D’Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Successful extrarenal transplantation from non-heart-beating donors. Transplantation 1995;59:977-82. [PubMed]
- Cypel M, Sato M, Yildirim E, et al. Initial experience with lung donation after cardiocirculatory death in Canada. J Heart Lung Transplant 2009;28:753-8. [PubMed]
- Erasmus ME, Verschuuren EA, Nijkamp DM, et al. Lung transplantation from nonheparinized category III non-heart-beating donors. A single-centre report. Transplantation 2010;89:452-7. [PubMed]
- Love RB. Perspectives on lung transplantation and donation-after-determination-of-cardiac-death donors. Am J Transplant 2012;12:2271-2. [PubMed]
- Mason DP, Brown CR, Murthy SC, et al. Growing single-center experience with lung transplantation using donation after cardiac death. Ann Thorac Surg 2012;94:406-11; discussion 411-2. [PubMed]
- De Oliveira NC, Osaki S, Maloney JD, et al. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg 2010;139:1306-15. [PubMed]
- Levvey BJ, Harkess M, Hopkins P, et al. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplantcollaborative. Am J Transplant 2012;12:2406-13. [PubMed]
- Puri V, Scavuzzo M, Guthrie T, et al. Lung transplantation and donation after cardiac death: a single center experience. Ann Thorac Surg 2009;88:1609-14; discussion 1614-5. [PubMed]
- de Antonio DG, Marcos R, Laporta R, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant 2007;26:529-34. [PubMed]
- Bonser RS, Taylor R, Collett D, et al. Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet 2012;380:747-55. [PubMed]
- Sabashnikov A, Patil NP, Mohite PN, et al. Influence of donor smoking on midterm outcomes after lung transplantation. Ann Thorac Surg 2014;97:1015-21. [PubMed]
- Shigemura N, Toyoda Y, Bhama JK, et al. Donor smoking history and age in lung transplantation: a revisit. Transplantation 2013;95:513-8. [PubMed]
- Taghavi S, Jayarajan S, Komaroff E, et al. Double-lung transplantation can be safely performed using donors with heavy smoking history. Ann Thorac Surg 2013;95:1912-7; discussion 1917-8.
- Taghavi S, Jayarajan SN, Komaroff E, et al. Single-lung transplantation can be performed with acceptable outcomes using selected donors with heavysmoking history. J Heart Lung Transplant 2013;32:1005-12. [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [PubMed]
- Carrel A, Lindbergh CA. The culture of whole organs. Science 1935;81:621-3. [PubMed]
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [PubMed]
- Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg 2009;87:255-60. [PubMed]
- Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant 2009;9:2262-9. [PubMed]
- Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319-25. [PubMed]
- Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6. [PubMed]
- Yeung JC, Cypel M, Machuca TN, et al. Physiologic assessment of the ex vivo donor lung for transplantation. J Heart Lung Transplant 2012;31:1120-6. [PubMed]
- Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [PubMed]
- Cypel M, Aigner C, Sage E, et al. Three center experience with clinical normothermic Ex Vivo lung perfusion. The Journal of Heart and Lung Transplantation 2013;32:S16.
- Zych B, Popov AF, Stavri G, et al. Early outcomes of bilateral sequential single lung transplantation after ex-vivo lung evaluation and reconditioning. J Heart Lung Transplant 2012;31:274-81. [PubMed]
- Boffini M, Ricci D, Barbero C, et al. Ex vivo lung perfusion increases the pool of lung grafts: analysis of its potential and real impact on a lungtransplant program. Transplant Proc 2013;45:2624-6. [PubMed]
- Andreasson A, Karamanou DM, Perry JD, et al. The effect of ex vivo lung perfusion on microbial load in human donor lungs. J Heart Lung Transplant 2014. [Epub ahead of print]. [PubMed]
- Sanchez PG, Davis RD, D’Ovidio F, et al. Normothermic ex vivo. lung perfusion as an assessment of marginal donor lungs - the NOVEL lung trial. J Heart Lung Transplant 2013;32:S16-7.
- Sanchez PG, Davis RD, D’Ovidio F, et al. The NOVEL Lung Trial One-Year Outcomes. J Heart Lung Transplant 2014;33:S71-S2.
- Machuca TN, Cypel M, Keshavjee S. Advances in lung preservation. Surg Clin North Am 2013;93:1373-94. [PubMed]
- Wallinder A, Ricksten SE, Silverborn M, et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case-control study. Eur J Cardiothorac Surg 2014;45:40-4; discussion 44-5. [PubMed]
- Henriksen IS, Møller-Sørensen H, Møller CH, et al. First Danish experience with ex vivo lung perfusion of donor lungs before transplantation. Dan Med J 2014;61:A4809. [PubMed]
- Warnecke G, Moradiellos J, Tudorache I, et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lungbefore bilateral transplantation: a pilot study of 12 patients. Lancet 2012;380:1851-8. [PubMed]
- Israni AK, Zaun D, Rosendale JD, et al. OPTN/SRTR 2012 Annual Data Report: deceased organ donation. Am J Transplant 2014;14 Suppl 1:167-83. [PubMed]
- Sanchez PG, Bittle GJ, Williams K, et al. Ex vivo lung evaluation of prearrest heparinization in donation after cardiac death. Ann Surg 2013;257:534-41. [PubMed]
- Nakajima D, Chen F, Yamada T, et al. Reconditioning of lungs donated after circulatory death with normothermic ex vivo lung perfusion. J Heart Lung Transplant 2012;31:187-93. [PubMed]
- Mulloy DP, Stone ML, Crosby IK, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results insuperior lung function. J Thorac Cardiovasc Surg 2012;144:1208-15. [PubMed]
- Gomez-de-Antonio D, Campo-Cañaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 2012;31:349-53. [PubMed]
- Moradiellos FJ, Naranjo JM, Córdoba M, et al. Clinical Lung Transplantation after Ex Vivo Evaluation of Uncontrolled Non Heart-Beating Donors Lungs: Initial Experience. J Heart Lung Transplant 2011;30:S38.
- Egan T, Blackwell J, Forrest L, et al. Evaluation of Human Lungs From Category 1 Non-Heart-Beating Donors (NHBDs) With Ex-Vivo Lung Perfusion (EVLP) in the U.S. Chest 2014;145:635A.