Nobelpharma, a new Japanese pharmaceutical company that only provides medicines for unmet medical needs
Introduction
When I founded Nobelpharma in 2003. I received the following advice from an older colleague, “When you greet your employees at the start of a new year, don’t speak in negative terms such as we live in uncertain times, and nobody can tell what will happen while the world is in a recession, but I still ask each of you to do your best. Even if it might not come to pass, encourage your staff to look forward to a bright future and reflect on the good events of the preceding year”. In this article I would like to convey a positive view of the future!
Nobelpharma specializes in R&D of pharmaceuticals for medical treatment, particularly the later phases of clinical trials in Japan, application, corresponding regulatory review, obtaining approval, and following approval, price negotiations and sales. Within the entire field of pharmaceuticals, we only handle drugs for unmet medical needs.
Making only essential drugs
Nobelpharma’s clear mission (Table 1) is to “Contribute to medical care by providing drugs that are needed but not available, in other words, drugs for unmet medical needs.”

Full table
Once each quarter of the year all employees gather, and I speak to them about the current business situation and issues, and I always begin by talking about our company’s mission. “We will contribute to medical care by developing drugs for unmet medical needs that other companies do not pursue. We will not do anything else.” This is what I insist upon, and I am also adamant that when on some occasions we lose track of the appropriate direction, if we return to our mission, we can’t go wrong.
Utilizing the strengths of a small number of experienced and highly qualified personnel
“Drugs for unmet medical needs” does not provide a focus on any particular field of treatment, but we concentrate on pediatric, gynecological, and hereditary diseases, and on rare cancers.
We do not seek to become larger because we want to achieve fast decision making in a small organization. We have about 220 employees, about 120 of them are sales personnel, and 50 are working in R&D. We have obtained market approval for eight drug products since the company was established ten years ago. Many of our employees are veterans of major pharmaceutical companies who have a track record of success. They know, based on their experience, how to develop products efficiently in a short period of time. The average age of our employees is high, over 50 years of age.
Nobelpharma’s product development
Nobelpharma obtained approvals for three drugs in the first round of development, from 2003 to 2008. We selected three candidate products that we considered would have a high likelihood of obtaining approval. I believed that these three products would offer us the chance to rapidly bring them to market.
The first product that we developed was Nobelzin. This is a treatment for Wilson’s disease, a hereditary copper metabolic disorder, and we developed it in response to requests from patients’ organizations and related academic medical groups. There are about 1,000 to 3,000 patients in Japan, and we applied for approval after conducting phase III trials with 37 patients. We obtained approval in January 2008, the drug price was listed in April, and we launched sales. We obtained orphan drug designation at the beginning of development, and received assistance to cover 21% of development costs. However, post-marketing expenses such as for collection of safety data were significant, and this product has hardly been profitable, but we continue to sell it. Alfresa Pharma handles the drug marketing.
Our second product was Lunabell for gynecological patients. This is a drug to treat dysmenorrhea. Dysmenorrhea can cause unbearable pain and discomfort, significantly reducing the quality of life for young women. There were strong requests to develop this from patients’ and related academic groups, and it was even taken up in the Japanese Diet, but since there was a similar drug available at a very low price, no company would pursue it. Even though there was much clinical research reporting on its efficacy, there were no rigorous comparative trials worldwide, so we made our application after conducting a preliminary trial with 36 patients, a placebo-controlled Phase III trial with 96 patients, and a long-term dosage trial with 128 patients. Lunabell was approved in April 2008, the drug price was listed in June, and we launched it in July 2008, marketed by Nippon Shinyaku and Fuji Pharma. This drug is our major product, accounting for a large part of our sales, and we have continued to conduct life cycle management for it after the initial launch, by obtaining approval for additional indications and developing improved drugs to reduce adverse effects that were approved in June 2013. I believe it would not be possible to build a strong foundation for a company in this industry in Japan today with just orphan drugs and without one commercially successful product like this one.
The third product was Nobelbar, a drug for treatment of pediatric neurological patients. It is an intravenous injection used to treat neonatal seizures and status epilepticus, and it was developed in response to requests from academic neurological and pediatric societies. Prior to Nobelbar there were only agents for muscular injection that were available, which were not really appropriate for infants and newborn babies. Nobelpharma developed this drug on its own, and utilized Japanese government funding for R&D to conduct investigator-initiated trials. It was approved in October 2008, the drug price was listed in December, and then Alfresa Pharma launched the sale of the drug. This treatment for neonatal seizures was given orphan designation early in its development, and 24% of development expenses (other than for clinical trials) up until application were covered by additional aid from the Japanese government. However, this drug is not given repeatedly so sales are limited, and of course it is necessary to collect safety data so it is hardly profitable, but nevertheless patients continue to benefit and sales continue.
Obtaining approval of these three drugs made 2008 a wonderful year for our company. There were articles in the Asahi Shimbun and Nikkei Business about us, and overseas the pharmaceutical journal Script wrote that we had obtained marketing approvals for three products. Surprisingly, Nobelpharma was ranked first in Japan that year among Japanese pharmaceutical companies for the number of approved new drugs!
After that in our second round of development we obtained approval for Fostoin, a treatment for status epilepticus and for prevention of convulsive seizure after surgery, for Gliadel, a drug to treat malignant glioma, for Alabel, a diagnostic agent for visualizing the structure of malignant glioma during surgery, and for Unitalc, a drug to treat malignant pleural effusion. We were proud that our ability to introduce drugs from major established companies like Pfizer (Fostoin) and Eisai (Gliadel) confirmed the recognition of our development capabilities. We could not take on high risk initially when our company was established, so in principle we selected products for development that we saw had potential to be profitable for our company and that had already been approved overseas, but for whatever reason had not yet been approved in Japan. There were some products that did not fit our situation during our early years and which we were unable to introduce, but through perseverance and continuous negotiations, we were able to introduce some of them. Currently we have four products in the application process, and a number in development, including some that are completely new products, having never been approved in Japan or overseas. Some products that we are co-developing with universities were R&D agents that they produced, and we are receiving maximum public aid intended for venture companies in order to minimize development expenses, as part of our constant effort to find ways to be profitable.
We have specialized staff members assigned to discover and select products for development, a considerable number in light of the limited size of our company. These staff members meet regularly to evaluate the progress and status of each candidate product. They each have their own area of expertise and evaluate candidates accordingly, and I believe they do so quickly and accurately. Now we are finding fewer products with a similar low level of risk compared to those we found when the company was newly established, but it is vital for us to find new promising candidate products. I myself am also positively involved in selecting the right products to fulfill our mission, and I work hard to introduce the ones that I decide are right (Table 2).
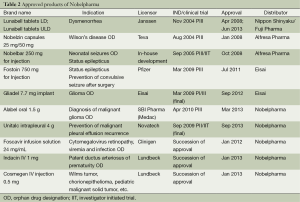
Full table
Financing by business investors and from banks
From the very start we did not use venture capital for financing, but obtained funds from Inabata & Co. Ltd. and from a number of banks. Based on the company’s mission, the goal was not to grow, but rather to contribute to society, and our management policy was to obtain sales and profits as a means to that end. Our objective was not to have shares listed, and so it was not possible to obtain capital from venture capitalist investors that would look for stock ownership and capital gains on the stock market. Inabata agreed with our company’s mission and supported us financially from the very start with both investment and loans. Development Bank of Japan Inc. invested in preferred stock, and various governmental financial institutions, business investors, and commercial banks made long-term loans to us, including The Bank of Tokyo Mitsubishi UFJ Ltd., and Mizuho Bank Ltd.
The attraction of drugs for unmet medical needs
Sometimes I am asked why we develop drugs for unmet medical needs. First, it is the attractiveness of developing drugs that are needed. It is usually the case that there are strong requests from the patients to develop them, and so there are many opportunities to meet the patients directly, and we hear straight from the patients and their families. We always feel our responsibility during development, and when we obtain an approval, the feeling of success is so much greater than for ordinary drug products.
The second reason is that drugs for unmet needs are usually profitable to some degree. However, in Japan the drug price is determined after approval is obtained, and if the sales forecasts were wrong or if the desired drug price is not obtained, then it is possible to lose money.
The third reason is Pharmaceuticals and Medical Devices Agency (PMDA)’s attitude towards drugs for unmet medical needs. PMDA has a different attitude toward the drugs that we are developing than towards ordinary drugs, for which they may not care about their ultimate presence or absence in the market. They recognize that these products are to answer unmet medical needs.
There are examples in the US of companies that succeeded in business as specialists in the medical field, but that is not the case in Japan. Genzyme is a famous example, founded in 1981, and now doing business in 40 countries around the world, with 10,000 employees and sales of around $4.5 billion. It became part of the Sanofi group in 2011.
There are a number of reasons that a company like Genzyme would not be born in Japan, but I think that the biggest are R&D capability, financing capability, and the drug pricing system. Among those reasons, drug pricing stands out. In the US, companies may set their prices freely, but in Japan the government sets the price for the National Health Insurance system after the drug is approved, and even if companies have some room to negotiate, the government has unconditional authority to set the price. This drug pricing system is sometimes unusually callous towards orphan drugs. Despite the fact that these drugs are completely different from those for treating high blood pressure, or hyperlipidemia with nearly ten million patients, and will be very little burden on health insurance finances, they hold prices down to the point where it becomes very difficult to make a profit at all. Consequently, even after working hard to develop an orphan drug, you can’t make enough profit to reinvest in development of the next product. It is because companies in Japan cannot obtain an adequate price for products originally developed here that products are developed in Japan only after they are developed in a country with a freer drug pricing system, especially in the US. It is for these reasons that it is so difficult to establish a company like Genzyme, specializing in orphan drugs, in Japan. While it may be that drug prices are supported by taxes, if you do not allow pricing that rewards excellent innovation, then you will not have people who can create innovative products, and it will be difficult to foster innovation. Currently, new drug products developed in Japan are priced low both in Japan and overseas. The result is that it does not benefit Japan.
Setting higher drug prices for excellent innovation that provides unique benefits to needy patients would mean accepting higher-priced medical care, but in a wealthy modern country like Japan I do not think there are many people opposed to using national finances in such a manner. Our country is poor in natural resources, and in order for us to continue our current prosperity, I believe that our government must amend its way of thinking.
Development overseas
So far most of the drugs our company has had approved were already approved in the US or Europe and were introduced from there, but recently, as I mentioned earlier, we have begun to accept the challenge of developing new drug ideas. It is impossible to avoid some failures in the course of drug development, and so we cooperate with overseas companies to spread the risk, emphasize cooperation with universities, and keep an eye on international development as much as possible. In the near future we will conduct international joint clinical trials, and are thinking about the possibility of Japanese candidate products to obtain approvals in the future in neighboring countries, Europe, or the US.
The kind of people that Nobelpharma requires
Our company exists in order to “develop and sell drugs that must be provided.” If we did not have people who had experienced success and who have multiple capabilities, we could not succeed. In addition, more than anything else, we need people who have a strong desire to develop the drug products that are truly required in the medical field to improve patient outcomes.
I work hard to create an environment where employees can work with a good feeling about their job, but our company is only ten years old so compensation is lower than that in major companies, and although I think it is regrettable, sometimes there is a lot of overtime work. However, over these past years there have been very few who have quit working here, and we have a good employee retention ratio.
The wonderful thing about drugs for rare diseases
I am happy that we have developed drugs for rare diseases when patients and doctors express their gratitude. That is the greatest joy for us.
Acknowledgements
Disclosure: The author declares no conflict of interest.


