Home-based exercise: promising rehabilitation for symptom relief, improved functional status and quality of life for post-surgical lung cancer patients
Individuals with non-small cell lung cancer (NSCLC) face difficult challenges in symptom control and management on a daily basis (1). In fact, troublesome symptoms are often present at the time of diagnosis with NSCLC (2). Unfortunately, NSCLC treatment may not always relieve the symptoms that patients endure (3). Instead, treatment may initiate an onset of new symptoms and/or exacerbate the severity and distress of existing symptoms (4). Furthermore, lung cancer often presents in older populations that have a history of tobacco dependence and other co-morbid conditions such as hypertension, heart disease, and chronic obstructive pulmonary disease. Co-morbidities are often accompanied by symptoms that worsen because of cancer and its treatment (5). Unfortunately, concomitant symptoms from cancer, cancer treatment, and co-morbidities persist throughout cancer treatment and further negatively affect the functional status and quality of life (QOL) of individuals with lung cancer (1). Moreover, individuals with lung cancer are less likely to be directed to rehabilitation programs for self-management or defined recovery regimens (6-9). As a result, symptom self-management strategies need to be developed and tested in order to preserve and maximize the functional status and QOL of individuals with lung cancer. The purpose of this pilot study was to describe the effects of a post-surgical home exercise intervention implemented immediately after hospital discharge on cancer-related fatigue (CRF), other symptoms, functional status, and QOL in individuals with NSCLC.
Background
Thoracotomy provides the best treatment for potential cure of individuals with early stage NSCLC (10). However, symptom knowledge related to symptoms, functional status, and QOL outcome research following thoracotomy is limited, and the reports are not optimistic.
Cancer-related fatigue (CRF)
CRF is a debilitating symptom that is infamous for its prevalence, severity, and persistence causing a profound, negative effect on functional status and QOL (11). As defined by the National Comprehensive Cancer Network, CRF is a distressing, persistent, subjective sense of tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning (12). Individuals with cancer report that CRF is the most distressing symptom related to their cancer and cancer treatment (13-15). Likewise, a single underlying cause to CRF has eluded researchers complicating CRF screening and leaving health care professionals puzzled in their ongoing efforts to address this symptom (12). The 2013 National Comprehensive Cancer Network Guidelines reiterated that screening practices for CRF in the clinical setting are not systematic or effective for a variety of reasons leaving CRF to be underreported, under-diagnosed, and under-treated (12). As a result, CRF is commonly known to be surrounded by a constellation of symptoms interfering with a person’s functional status and QOL (16).
Symptoms
For individuals undergoing thoracotomy for NSCLC, limited symptom information exists spanning the period from diagnosis to immediately after thoracotomy and throughout the recovery trajectory. What is known regarding post-operative recovery in this population suggest that patients suffer from myriad of symptoms that are multifactorial and attributed to the cancer, cancer treatment, and existing co-morbidities (17,18). Moreover, symptoms have been found to be severe and persistent for post-thoracotomy patients (7,17,18). Also, symptoms have been found in clusters for post-thoracotomy NSCLC patients and the clusters include the symptom of fatigue (7,18). Consequently, symptoms place an additional burden on the lives of individuals post-thoracotomy for lung cancer with multiple symptoms reported as severe and persisting well into the post-surgical recovery period and beyond. Unfortunately, unmanaged fatigue and other symptoms have a direct negative effect on functional status and QOL (3,16).
Functional status
Few longitudinal studies exist that measure changes in functional status and QOL for post-thoracotomy NSCLC patients. Kurtz reported that symptom severity was a significant predictor of physical functional status (19). One symptom, fatigue, has been reported to be an independent predictor of physical functioning (20). Moreover, CRF has been found to have a negative direct effect on physical functioning and a negative indirect effect on physical functioning by worsening the severity of other symptoms in individuals with cancer including lung cancer (16) Furthermore, post-operative declines in physical and mental functional status scores of at least 10% have been found to be associated with an 18% and 13% higher risk of mortality, respectively (21). Among an age-matched healthy population, pre-operative lung cancer patients not only had worse physical functioning, but they reported worse mental functional status scores than their healthy counterparts (3). Six months post-operatively when compared to the age-matched healthy population, individuals with lung cancer showed continued worsening in the area of physical and mental functional status (3). Thus, findings demonstrated that pre-operative functional status in individuals after lung resection is impaired, and impairment often persists over time (22). Consequently, individuals with lung cancer often express concern about living with the aftermath following surgical resection (23).
Quality of life (QOL)
While people are concerned with how long they are going to live they are also concerned with how well they are going to live in terms of their functional status and the QOL. Most research on post-thoracotomy patients focuses on post-surgical morbidity, mortality, and survival. When QOL has been investigated within the post-surgical NSCLC population, surrogate instruments such as those focused on functional health status combined with varying symptom assessments are typically used to characterize QOL. Using a QOL measure, the Quality of Life Index (QLI), Handy found that diffusion capacity of the lung for carbon monoxide was a predictor for a reduced level of QOL at six months post-thoracotomy (3). Likewise, Handy recommends further exploration of symptoms relative to QOL because in this study pain and dyspnea worsened six months post-thoracotomy (3). Likewise, in two studies conducted by Schulte, post-thoracotomy NSCLC patients failed to make a complete recovery over a 24-month period where neither younger (<70 years of age) nor older (>70 years of age) patients returned to pre-operative QOL levels (22,24). Expansion of research is needed to document QOL in earlier stage lung cancer prior to surgery, immediately after surgery, and on a periodic basis through the recovery trajectory.
Exercise and CRF
With CRF and other symptom severity strongly associated with functional status and QOL, developing an intervention addressing CRF for the post-thoracotomy NSCLC population was a priority of our research team. To date, exercise has been found to be an effective treatment for CRF (25); however, there is little evidence to support the timing, type, frequency, duration, and intensity of exercise for this population (26,27). In July 2010, the American College of Sports Medicine convened a panel of cancer and exercise specialists to develop guidelines for cancer survivors (28). The guidelines conveyed an important message for cancer survivors: avoid inactivity, and return to normal daily activities as quickly as possible after surgery. Specific issues outlined as challenges to exercise by this panel included patients with co-morbidities, decreased range of motion, and/or substantial deconditioning. All of these are major challenges faced by the post-surgical NSCLC population. This article reports results of the preliminary efficacy of the first home-based, light-intensity exercise intervention implemented immediately post-discharge from the hospital after thoracotomy that targeted CRF and its effect on other symptoms, functional status, and QOL in this vulnerable NSCLC population.
Methods
Design, setting, and sample
This pilot study assessed patterns of change in CRF, other symptoms, functional status, and QOL from pre-surgery to the end of a 6-week home exercise program. Measures were obtained prior to surgery, post-surgery at first home visit when the exercise program had begun, and at weeks 3 and 6 of the exercise program. The study was approved by the institutional review boards at Michigan State University, Grand Valley State University, and a university teaching hospital in Michigan. Enrollment commenced in March 2011 with participants being recruited from two sites at the university teaching hospital. Individuals at least 21 years of age with suspected NSCLC were eligible to participate with: (I) a Karnofsky Performance Status Score at least 70% before surgery; (II) medical clearance from thoracic surgeon after surgery; (III) stable co-morbid conditions; and, (IV) willingness to follow health and safety rules. Participants were excluded with: (I) severe visual, hearing or speaking deficits; (II) oxygen therapy for activities of daily living; (III) weight greater than 300 pounds; (IV) photosensitive seizures or diagnosed dementia; and (V) conditions hindering safe participation. Thirteen individuals were invited to participate, and seven agreed. All seven completed the 6-week exercise intervention.
Pre-intervention procedures
An oncology registered nurse recruiter identified and recruited participants meeting the eligibility criteria. After signing informed consent, participants completed baseline questionnaires before the start of the intervention.
Intervention
After study enrollment, a registered nurse intervener (nurse) taught participants to self-manage CRF using the National Comprehensive Cancer Network Guidelines and to participate in exercise after hospital discharge. Within 72 hours of discharge from the hospital, the nurse performed an assessment of core symptoms (pain, nausea, vomiting, and dyspnea) to ensure exercise readiness. Once adequate symptom control was confirmed, the nurse conducted the first home visit (mean time from hospital discharge to first home visit was 66 hours, range 22-100 hours) and implemented the exercise protocol, which included warm-up exercises and light-intensity (less than 3.0 metabolic equivalents) walking and balance exercises with the Nintendo Wii. Walking started at 5 minutes per day for 5 days a week during week 1 and increased toward the goal of 30 minutes a day at the start of week 6. Participants achieved a mean of 26 (SD, 4.6) minutes per day during the last week of the exercise intervention. Walking duration was increased 5 minutes a day each week if the participant’s confidence for achieving a specified duration was 70% or greater on a 0- to 100-point scale, with 100% having high confidence. Participants also completed balance exercise 5 days a week for the study duration. Within 24 hours after the first home visit, the nurse made a phone visit to answer any questions about the exercise intervention. At the start of week 2, the nurse assessed the exercise prescription via a home visit with subsequent phone visits at the start of weeks 3 to 6. Additional home visits by the nurse were available upon participant request.
Additional details about the intervention components, safety, and feasibility have been reported elsewhere (29).
Outcome measures
CRF severity was measured using the Brief Fatigue Inventory (BFI) (30). Nine items were rated on an 11-point scale (0-10, 10= most severe) focusing on CRF severity at its worst in the past 24 hours. Reliability and validity of the BFI is well established. The Cronbach α for this study ranged from 0.89 to 0.98.
Symptom severity and interference were measured using the M.D. Anderson Symptom Inventory Core and Lung Module (MDASI) (31). The MDASI measures the severity of 16 symptoms and 6-items measure the interference from symptoms on daily living at its worst in the past 24 hours. In this study, assessment of fatigue was excluded with the use of the MDASI. The 15 items were rated on an 11-point scale (0-10, 10= most severe and most interference). The Cronbach α for the study ranged from 0.71 to 0.92 with most scores .84 or greater with scores less than 0.70 at week 5 (Cronbach α, 0.67).
Functional Status was measured using the well-established Medical Outcomes Short Form-36 Version 2 Acute Recall (SF-36) (32). Principle component analysis demonstrates that 80-85% of the variance in the eight subscales was accounted for by two factors, physical and mental health. The subscale scores of the SF-36 are linearly transformed providing normative scores from 0 to 100, with higher scores representing better levels of functional status (32). Similar to reported reliabilities, internal consistency reliability for the Physical Health Component (PHC) summary scale ranged from 0.78 to 0.90 except for week 3 (Cronbach’s α, 0.61) of our study. Likewise, internal consistency reliability for the Mental Health Component (MHC) summary scale ranged from 0.78 to 0.92 except for week 3 (Cronbach’s α, 0.51).
QOL was measured using Ferrans and Powers QLI (33-35). The QLI is a two-part questionnaire with reliability and validity established in the cancer population. The QLI assesses both satisfaction and importance of aspects of life to the person. Each part is comprised of 32 items that assess overall QOL via the following four domains: health and functioning, social and economic, psychological and spiritual, and family. Participants respond to a 6-point rating scale ranging from very dissatisfied to very satisfied for the satisfaction items and from very unimportant to very important for the importance items. Scores for the QLI range from 0 to 30, with higher scores indicating better QOL.
Participant characteristics were obtained from a demographic questionnaire and a medical chart review.
Statistical analyses
Analyses were completed using IBM SPSS Statistics version 19.0 (36). The patterns of change in CRF and symptom outcomes were longitudinally graphed from pre- and post-surgery and at the end of each week of the 6-week intervention. Likewise, results for functional status and QOL outcomes were graphed pre- and post-surgery, at week 3, and at the end of the intervention (6 weeks).
Results
Five women and two men (mean age 64.6; SD 6.5; range 53-73 years) participated, and all underwent a thoracotomy for a lobectomy for NSCLC. Stage of cancer ranged from IA to IIIA. Participants had on average 5.9 (SD 3.4; range 2-12) co-morbid conditions.
Cancer-related fatigue and other symptoms
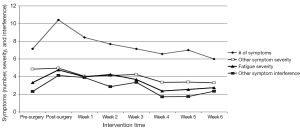
Figure 1 shows the overall pattern of symptom mean scores from pre-surgery to week 6 of the exercise program. On average, participants experienced seven symptoms pre-surgery that increased to ten symptoms post-surgery and declined to six symptoms by week 6. CRF severity increased from 3.5 to 4.8 pre- to post-surgery and then decreased to 2.8 by week 6. Other symptom severity went from 4.9 to 5.0 pre- to post-surgery and decreased to 3.5 by week 6. Other symptom interference increased from 2.5 to 4.1 pre- to post-surgery and decreased to 2.2 at week 6. Five of seven participants started chemotherapy at week 5 of the home exercise program.
Functional status
Physical functional status
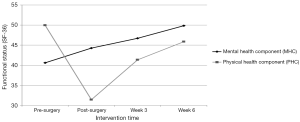
As seen in Figure 2, the MOS-36 PHC score indicating overall physical functional status pre-surgery was 49.8, decreased to 31.5 post-surgery, and increased to 41.4 and 45.9 at weeks 3 and 6, respectively. Consistent with the overall PHC mean score, all physical functional status subscale mean scores (physical functioning; role-physical functioning; bodily pain, and general health) decreased post-surgery and increased by the end of weeks 3 and 6.
Mental functional status
As seen in Figure 2, the MOS-36 MHC mean score indicating mental functional status pre-surgery was 40.6. Post-surgery and at weeks 3 and 6, the MHC mean scores rose to 44.3, 46.7, and 49.8, respectively. The patterns of change in mean scores of the mental functional status domains of vitality, social functioning, role-emotional functioning, and mental health were consistent with the overall MHC with each of the post-intervention scores increasing over pre-surgery scores: vitality (48.9 to 52.4); social functioning (41.9 to 52.5); role-emotional functioning (39.8 to 44.2); and mental health (41.8 to 47.8).
Quality of life (QOL)
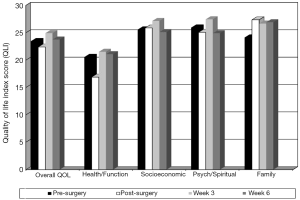
As seen in Figure 3, overall QOL scores decreased from 23.5 pre-surgery to 22.4 post-surgery and increased to a high of 24.9 at week 3 and decreased to 23.8 at week 6 post-intervention. Health and functioning domain score patterns pre-surgery, post-surgery, and at weeks 3 and 6 were 20.6, 16.9, 21.5, and 21.1, respectively. Psychological and spiritual domain means score patterns were 25.9, 25.1, 27.5, and 24.9, respectively. Socioeconomic domain score patterns were 25.6, 25.9, 27.2, and 25.1, respectively, while family score patterns were 24.1, 27.4, 26.7, and 26.9, respectively. All showed improvement at week 3 over the pre-surgical baseline and a slight decrease at week 6 with 5 out of 7 participants starting chemotherapy at week 5.
Hospital readmissions and unplanned acute care visits
After discharge from the hospital through week 6 post-intervention, there were no re-hospitalizations or unplanned acute care visits.
Discussion
In this first study to assess outcomes of a self-managed home exercise program in NSCLC patients immediately post-thoracotomy, participants mirrored those in previously reported research. They were older with a similar mean age of 65 years, and had a high number of co-morbid conditions (mean of 6). Given that the intervention was shown to be feasible and safe, the next critical step was to determine if positive outcomes in CRF, other symptoms, functional status, and QOL could be achieved in this high acuity population.
Prior to surgery, participants reported a high number of other symptoms (mean of 7) in addition to CRF that increased post-surgery. From post-surgery to week 6, there was an overall pattern of decline in CRF severity and other symptom mean scores with a slight increase after week 5 of the exercise intervention when chemotherapy was initiated on average for 5 out of 7 participants. By week 4, mean scores for CRF severity and severity and interference of other symptoms were below pre-surgical baseline mean scores. In addition, the average number of symptoms was below the pre-surgical baseline by week 6. These findings are similar to other reports of the number of symptoms and co-morbidities of individuals with NSCLC, but this is the first report showing improvement of symptoms with a home-based exercise program immediately post-thoracotomy.
Pre-operatively, the PHC and MHC representing overall physical and mental functional status were below the mean of 50 for the general U.S. population, 49.8 and 40.6 respectively. Post-surgically, as anticipated, the PHC score fell dramatically to 31.1. Conversely, the MHC score showed improvement post-surgery and continued to improve in weeks 3 and 6 surpassing pre-surgical scores finishing at 49.8 and approaching the general U.S. population mean mental functional status score of 50. Also, PHC scores showed marked improvement in both weeks 3 and 6 nearly achieving pre-surgical baseline levels at post-intervention (week 6) despite the start of adjuvant therapy for 5 of 7 participants at week 5. While research on functional status in the NSCLC population pre- and immediately post-surgery is limited; the findings in this study parallel those reports. However, this is the first study to demonstrate improvements in functional status through a home-based exercise program immediately after hospital discharge. Moreover, this is the first study to demonstrate improvements over the surgical treatment trajectory, establishing a trend from pre-surgery, immediate post-surgery after hospital discharge prior to initiation of exercise, after three weeks of the intervention, and later after a total of six weeks of the intervention.
Last, overall QOL scores decreased from pre-surgery to post-surgery and increased to their highest level exceeding pre-surgery scores at week 3. Despite 5 of 7 participants undergoing adjuvant therapy at week 5, overall QOL scores remained slightly above pre-surgery levels at post-intervention, week 6. Relative to the individual domains of QOL, at post-surgery, the health and functioning and psychological and spiritual domains of QOL worsened over pre-surgery scores while socioeconomic and family increased over pre-surgery scores. All domains of QOL exceeded pre-surgery levels at week 3 of the exercise intervention. The domains of health and functioning, socioeconomic, and family remained above pre-surgery levels at post-intervention while the spiritual domain of QOL dropped slightly below pre-surgery baseline. Therefore, nearly all of the improvements of the domains of QOL were sustained at the close of the exercise intervention (end of week 6) even as 5 of 7 participants started adjuvant therapy at week 5 suggesting that a home-based exercise intervention to self-manage CRF may have a positive effect on QOL for post-thoracotomy NSCLC patients. While little is known about the evolution of QOL in NSCLC patients who have undergone thoracotomy, the majority of studies incorporating QOL measures in individuals with lung cancer occur much later after surgery or during late stage disease. Also, symptom severity is closely related to QOL in patients with lung cancer and post-surgical NSCLC patients tend to deteriorate related to unmanaged symptoms such as CRF. This is the first known study to document the preliminary efficacy of a home-based exercise intervention for the severity of CRF and subsequent QOL. In addition, QOL measurements were taken regularly prior to surgery and throughout the immediate recovery period to assess the impact of the exercise intervention on QOL with preliminary results indicating positive effects on QOL.
This small pilot study was the first to implement a self-managed, home-based exercise program immediately upon hospital discharge after thoracotomy with resulting performance outcomes that were very promising in this challenging population. The design of this study captured timely, focused data to inform researchers and clinicians of the status of CRF, other symptoms, functional status, and QOL immediately before and after surgery upon hospital discharge for individuals with NSCLC. Likewise, focused assessment of CRF, other symptoms, and health care utilization were assessed on a weekly basis with assessment of functional status and QOL at regular intervals throughout the six-week post-surgical recovery period. Note that 5 out of 7 participants initiated chemotherapy on average at week 5 of the intervention. Further study using a comparative clinical trial design is warranted and is underway by the authors. With the promising efficacy demonstrated in this study, the potential clinical implications of this practical, home-based exercise program would be a major step forward for the recovery and rehabilitation of this population whose surgical treatment is aimed at cure of their NSCLC.
Acknowledgements
Funding: This work was supported by Michigan State University, College of Nursing, East Lansing, Michigan.
Disclosure: The authors declare no conflict of interest.
References
- Yang P, Cheville AL, Wampfler JA, et al. Quality of life and symptom burden among long-term lung cancer survivors. J Thorac Oncol 2012;7:64-70. [PubMed]
- Gift AG, Jablonski A, Stommel M, et al. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum 2004;31:202-12. [PubMed]
- Handy JR Jr, Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002;122:21-30. [PubMed]
- Akin S, Can G, Aydiner A, et al. Quality of life, symptom experience and distress of lung cancer patients undergoing chemotherapy. Eur J Oncol Nurs 2010;14:400-9. [PubMed]
- Given CW, Given B, Azzouz F, et al. Predictors of pain and fatigue in the year following diagnosis among elderly cancer patients. J Pain Symptom Manage 2001;21:456-66. [PubMed]
- Sanders SL, Bantum EO, Owen JE, et al. Supportive care needs in patients with lung cancer. Psychooncology 2010;19:480-9. [PubMed]
- Sarna L, Cooley ME, Brown JK, et al. Symptom severity 1 to 4 months after thoracotomy for lung cancer. Am J Crit Care 2008;17:455-67. [PubMed]
- Brunelli A, Socci L, Refai M, et al. Quality of life before and after major lung resection for lung cancer: a prospective follow-up analysis. Ann Thorac Surg 2007;84:410-6. [PubMed]
- Granger C, Denehy L. Exercise interventions following surgery for non-small cell lung cancer (NSCLC): the need for future randomised controlled trials. Lung Cancer 2010;70:228-9. [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Minton O, Berger A, Barsevick A, et al. Cancer-related fatigue and its impact on functioning. Cancer 2013;119 Suppl 11:2124-30. [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: cancer-related fatigue version 1.2013. Fort Washington, PA2012: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive, accessed November 1, 2013.
- Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol 2008;26:233-41. [PubMed]
- Braun DP, Gupta D, Staren ED. Quality of life assessment as a predictor of survival in non-small cell lung cancer. BMC Cancer 2011;11:353. [PubMed]
- Hung R, Krebs P, Coups EJ, et al. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. J Pain Symptom Manage 2011;41:426-35. [PubMed]
- Hoffman AJ, von Eye A, Gift AG, et al. Testing a theoretical model of perceived self-efficacy for cancer-related fatigue self-management and optimal physical functional status. Nurs Res 2009;58:32-41. [PubMed]
- Sarna L, Cooley ME, Brown JK, et al. Women with lung cancer: quality of life after thoracotomy: a 6-month prospective study. Cancer Nurs 2010;33:85-92. [PubMed]
- Brown JK, Cooley ME, Chernecky C, et al. A symptom cluster and sentinel symptom experienced by women with lung cancer. Oncol Nurs Forum 2011;38:E425-35. [PubMed]
- Kurtz ME, Kurtz JC, Stommel M, et al. Predictors of physical functioning among geriatric patients with small cell or non-small cell lung cancer 3 months after diagnosis. Support Care Cancer 1999;7:328-31. [PubMed]
- Given B, Given C, Azzouz F, et al. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nurs Res 2001;50:222-32. [PubMed]
- Möller A, Sartipy U. Associations between changes in quality of life and survival after lung cancer surgery. J Thorac Oncol 2012;7:183-7. [PubMed]
- Schulte T, Schniewind B, Dohrmann P, et al. The extent of lung parenchyma resection significantly impacts long-term quality of life in patients with non-small cell lung cancer. Chest 2009;135:322-9. [PubMed]
- Maliski SL, Sarna L, Evangelista L, et al. The aftermath of lung cancer: balancing the good and bad. Cancer Nurs 2003;26:237-44. [PubMed]
- Schulte T, Schniewind B, Walter J, et al. Age-related impairment of quality of life after lung resection for non-small cell lung cancer. Lung Cancer 2010;68:115-20. [PubMed]
- Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;11:CD006145. [PubMed]
- Thompson W. ACSM’s Guidelines for Exercising Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2010.
- Jones LW, Eves ND, Kraus WE, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010;10:155. [PubMed]
- Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409-26. [PubMed]
- Hoffman AJ, Brintnall RA, Brown JK, et al. Too sick not to exercise: using a 6-week, home-based exercise intervention for cancer-related fatigue self-management for postsurgical non-small cell lung cancer patients. Cancer Nurs 2013;36:175-88. [PubMed]
- Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186-96. [PubMed]
- Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 2000;89:1634-46. [PubMed]
- McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247-63. [PubMed]
- Ferrans CE, Powers MJ. Quality of life index: development and psychometric properties. ANS Adv Nurs Sci 1985;8:15-24. [PubMed]
- Ferrans CE, Powers MJ. Psychometric assessment of the Quality of Life Index. Res Nurs Health 1992;15:29-38. [PubMed]
- Ferrans CE. Development of a quality of life index for patients with cancer. Oncol Nurs Forum 1990;17:15-9; discussion 20-1. [PubMed]
- SPSS 15.0 [computer program]. Chicago, IL: SPSS Inc, 2008.


