|
Review Article
Acute pulmonary embolism after pneumonectomy
Zongfei Wang, Chu Pei, Lunchao Ma, Daoyuan Wang, Jiangfen Zhou, Wei Wang, Jianfei Shen, Zhiqiang Xu, Jianxing He
Department of Cardiothoracic Surgery, the First Affiliated Hospital of Guangzhou Medical College; Guangzhou Institute of Respiratory Disease, State Key Laboratory of Respiratory Disease, Guangzhou, China
Corresponding to: Jianxing He, MD, PhD, FACS. Department of Cardiothoracic Surgery, the First Affiliated Hospital of Guangzhou Medical College, No. 151, Yanjiang Rd, Guangzhou 510120, Guangdong Province, PR China. Tel: +86-20-83062800; Fax: +86-20-83062800. Email: drjianxing.he@gmail.com.
|
|
Abstract
Pulmonary embolism (PE) by occlusion of the pulmonary arterial bed may lead to acute life-threatening but potentially reversible right ventricular failure, one of the most severe complications of thoracic surgery. Still, the incidence of acute pulmonary embolism after surgery is reduced by comprehensive anticoagulant prevention, improved surgical techniques, appropriate perioperative management and early ambulation. However, there is difficulty in diagnosing PE after thoracic surgery due to the lack of specific clinical manifestations. So that optimal diagnostic strategy and management according to the clinical presentation and estimated risk of an adverse outcome is fundamental.
Key words
Acute pulmonary embolism; lung cancer; pneumonectomy J Thorac Dis 2012;4:76-82. DOI: 10.3978/j.issn.2072-1439.2011.10.02
|
|
Acute pulmonary embolism (PE) is one of the most severe complications in thoracic surgery. The occlusion of pulmonary arterial vascular bed may lead to sudden life-threatening danger and the potentially reversible right ventricular (RV) failure. It is very difficult to diagnose PE, and meanwhile the missed diagnosis and misdiagnosis are extremely common due to the lack of specific clinical manifestations ( 1). The mortality rate reaches up to 30% in un-treated PE patients, where as it may also be as low as 2%-10% in patients with timely diagnosis and treatment ( 2).
|
|
Epidemiology
The data of PE after thoracic surgery are extremely rare, and mostly case reports, which may be associated with its lower occurrence rate after thoracic surgery as compared with that after orthopedic, general and gynecological surgeries ( 3). Incidence of PE after thoracotomy reported by Collins in 1988 was up to 20% ( 4), and that by Ziomek in 1993, the most frequently cited in literature, was 5%, and the incidence reported by Dentali et al in 2008 through active prevention was 1.3% ( 6). Reduced incidence of PE after surgery is suggested along with comprehensive anticoagulant prevention, better surgical techniques, appropriate management in perioperative period and early ambulation, etc. Although video-assisted thoracic surgery (VATS) has been widely carried out, there is no relative literature and report on PE after VATS yet.
|
|
Risk factors and natural course
The risk factors of PE includes old age, previous history of venous thromboembolism (VTE), active tumor, nerve diseases accompanied with acromelic paralysis, surgeries, diseases involving prolonged bedridden time, natural or acquired thrombophilia, hormone replacement therapy and oral contraception, etc ( 1). Sorensen et al. ( 7) reported the high risk of PE in obese people, smokers and patients with systemic hypertension or metabolic syndrome (MS). Furthermore, there were some specific risk factors associated with pulmonary lobectomy. Ziomek et al. ( 5) reported that PE occurrence was higher in malignant tumor than in benign diseases, in primary bronchial cancer than in lung metastatic tumor, in adenocarcinoma than in other types, in lung cancer with diameter>3cm than in relatively smaller lung cancer, in II phase than in I phase and in pneumonectomy or pulmonay lobectomy than in segmentectomy or wedge resection. Kalweit ( 8) reported that the risk factors of PE after lung surgery included long surgery duration, chemo-treatment before surgery and little postoperative activity. Nagahiro et al. ( 9) pointed out that the higher PE occurrence in right lateral position (RLP) cases might be associated with iliac compression syndrome and decreased venous velocity of the right femoral vein. Moreover, the right lateral PE is more common due to gravity and increased blood flow in the right pulmonary artery after left pulmonary
lobectomy. Improper treatment of pulmonary stumps or anastomosis is also a risk factor for local thrombosis. Kwek et al. ( 10) confirmed that the length of stump is associated with thrombosis. The transfixing suture of pulmonary stumps causes thrombosis easily as compared with continuous suture in an
animal experiment by Isik et al. ( 11). PE and deep vein thrombosis (DVT) are two clinical
manifestations of venous thromboembolism (VTE) that involve
the same apt factors ( 1). DVT of upper or lower extremities
are found in 90% of PE patients, which suggests that PE is
the subsequent result of DVT in most cases ( 12). PE usually
occurs 3 to 7 days after DVT occurrence, 10% patients die
within one hour after presence of symptoms, whereas PE can
not be identified in most death cases by the current diagnostic
approaches. After PE occurrence, complete recoveries of the
perfusion defects can be found in about 2/3 patients. Most death
cases (>90%) were un-treated patients arising from diagnostic
failure of PE, while less than 10% of death cases underwent
treatment ( 1).
|
|
Diagnosis
It is much more difficult to diagnose PE after pulmonary
lobectomy than spontaneous PE, that’s because the clinical signs
and symptoms of PE such as chest pain, shortness of breath,
tachycardia and decreased blood oxygen saturation can be
considered to be correlated with incisional pain, reduced blood
volume and pulmonary atelectasis caused by the surgery or
covered by the analgesic measures such as an epidural anesthesia
( 3), hence it is necessary to indentify PE with pneumonedema,
heart failure, lung infection and bronchospasm after surgery.
Sakuragi et al. ( 13) suggested particular attention to the first
ambulation since most postoperative patients caught PE on
their first walk after operation. PE is highly suspected in patients
with symptoms such as sudden or progressively worsened
breathing difficulties, chest pain and persistent hypotension
( 14). Nevertheless, only 20% of patients are confirmed diagnosis
through objective workups ( 15). Although the individual
symptoms, signs and the conventional workups lack the
sensitivity and specificity, by a definite clinical prediction rule
in combination of these variables, more accurately diagnosis of
PE is still achievable. The latest guideline suggests that clinical
probability should be evaluated before diagnostic workups
( 16). The Wells Clinical Prediction Rule established by Wells
et al. ( 17) is the most commonly used rule ( Table 1), which
has been widely validated by three-fold classification (clinical probability of low, intermediate risk and high risk) and two-fold
classification (PE possibility). Since Wells Clinical Prediction Rule is somewhat not that
objective, more objective regulations are further established in
recent years, such as Geneva, Charlotte and Miniati rules, which
contribute to a more effective and simpler diagnosis of PE for
clinicians. The diagnosis is established on the clinical symptoms
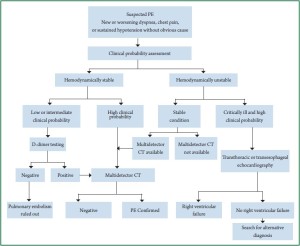
resulting from Haemodynamic stability or Clinical probability
assessment instability ( 14) ( Figure 1). D-dimer is a soluble degradation production generated
from cross linked fibrin based on the fibrinolytic system, with
negative prediction value higher than positive prediction value
( 1). Surgery, tumor, pregnancy, hospitalization and advanced
age might result in the elevated D-dimer ( 18). The quantitative
enzyme-linked immunoabsorbent assay (ELISA) showed
that the thromboembolic risk was 0.14% in patients with a
normal D-dimer concentration within 3 months of not taking
anticoagulation therapy ( 19). Therefore, the positive D-dimer
is of little significance in diagnosis of PE after pulmonary
lobectomy, while the negative D-dimer plays an important role
in acute PE exclusion hence no need of further workups for PE
diagnosis. Of course, the blood test be useful as first level exam,
especially for low/medium probability of clinical testing of
patients is of great significance. Been widely used in clinic, the chest multi slice CT pulmonary
angiography (SCTPA) shows direct signs of pulmonary arterial
low-density filling defect, partly or completely surrounded by the opaque blood flow or total filling defect with the distal end
of blood vessel being absent. The SCTPA has advantages such
as specificity of high spatial/temporal resolution and arterial
opaqueness, non-invasiveness, convenience and rapid result
acquisition. A large prospective research demonstrated that
the sensitivity of SCTPA was 83%, specificity was 95% ( 20)
and sensitivity to the emboli in pulmonary artery was 97%
( 21). Meanwhile, SCTPA displays abnormalities such as lung
infection, pulmonary atelectasis, pneumonedema and pleural
effusion, and assists making differential diagnosis of PE. So
that in clinical practice, SCTPA has been a preferred method
for pulmonary angiography in patients with suspicious PE. The
combined pulmonary CT angiography and CT venography of
lower extremity improves sensitivity and specificity (83%-90%,
95%-97%, respectively) ( 20), however, it should be avoided due
to its little clinical significance and high dose of radiation ( 22).
Dual-source CT (DSCT), first used in 2006, is fast, accurate and safe in diagnosing PE. DSCT enjoys a higher time resolution,
better spatial resolution and less radiation quantity compared
with single-source CT (SSCT) ( 2), and it is still developing
along with new technology and improved capacity ( 23). False
negative values by CT in PE patients with high clinical PE
probability are reported, however this situation is rare and these
patients have small risk of venous thrombosis within 3 months
after lobectomy. At present, it is still controversial on whether
to perform further examinations and on perform what kind of
examination on these patients ( 1). Pulmonary ventilation--perfusion scanning remains an
effective way for diagnosing patients with contraindications of
CT examination such as allergic to contrast agents, with renal
failure or pregnancy, in severe conditions or child-bearing age.
PE is excluded if pulmonary perfusion defects distributed in lung
segments do not match the ventilation imaging, and the normal
negative predictive value of ventilation-perfusion scanning is 97% ( 24). Nevertheless, the ventilation-perfusion scanning is
only applicable in diagnosis of 30%-50% suspicious PE patients.
A randomized contrastive study of CT and ventilation-perfusion
scanning showed that the occurrence rates of VTE were 0.4%
and 1.0% in patients excluded from PE by examinations within
3 months, respectively ( 25). Meanwhile, many diseases affect
both ventilation and blood flow, thus the ventilation-perfusion
scanning has to be interpreted according to the clinical status.
At present, the ventilation- perfusion scanning is only used in
patients with renal failure or allergy to contrast medium and
pregnant women. Echocardiography allows bedside operation and is used
in evaluation of right ventricular function, which has 96% of
sensitivity and 83% of specificity in diagnosis of PE combining
with clinical symptoms and electrocardiogram (ECG).
Thrombus in the pulmonary trunk is detected in PE patients with
hemodynamic instability by transesophageal ultrasonography
( 26). Echocardiography is the best option for PE patients at
high risk ( 27), it is mainly used in risk stratification though its
significance in diagnosis of non-high risk PE is little, and its
negative results may not exclude the possiblity of PE. Since 90% of PE are caused by deep vein thrombosis (DVT)
of lower extremities, ultrasonography on the lower extremity has
replaced venography in diagnosis of deep venous thrombosis
to a great extent. Based on proximal deep venous thrombosis, if
the sensitivity is over 90% and the specificity is about 95%, 10%
patients would not need for further lung scanning.
Magnetic resonance angiography (MRA) refers to a
novel diagnostic technique that can be used in patients with
contraindications of iodine contrast agent or ionizing radiation.
A prospective Phase III study ( 29) showed sensitivity and
specificity of being 78% and 99% respectively when excluding technique improper (25%). The sensitivity can be increased
to 92% in combination with magnetic resonance venography
(MRV), whereas the proportion of improper technique also
increases to 52%. Compared with SCTPA, MRA takes a longer
time, poorer acceptability for patients, also it fails in excluding
other cardiovascular and lung diseases apart from PE, besides
it is not available for patients with implanted pacemaker and
other equipments. MRA is complex, so MRA is only suggested
in patients with contraindications to standard workups in
conventional medical centers. The catheter pulmonary angiography is the gold standard
in diagnosing PE, with sensitivity at 98% and specificity at
95%-98%. The direct signs are the pulmonary filling defects or
complete obstruction of pulmonary artery branches, according
to which, small thrombosis of 1-2 mm in length are visible in
segmental pulmonary artery ( 30). Nevertheless, the catheter
pulmonary angiography is usually used before skin embolus
resection since it is invasive and expensive, and it might lead to
complications such as increased local bleeding associated with
thrombolytic therapy (TT) and even death in severe patients. In sum, the examination approaches should be selected
according to the clinical probability, patient status, applicable
examination approaches, allergic to iodine contrast agent or not,
risk of ionizing radiation and examination costs, etc.
|
|
|
| Table 1. Wells Clinical Prediction Rule. |
| Variable |
Points |
| Predisposing factors |
| Previous DVT or PE |
+1.5 |
| Recent surgery or immobilization |
+1.5 |
| Cancer |
+1 |
| Symptoms |
| Haemoptysis |
+1 |
| Clinical signs |
| Heart rate>100 beats/min |
+1.5 |
| Clinical signs of DVT |
+3 |
| Clinical diagnosis |
| Alternative diagnosis less likely than PE |
+3 |
| Clinical probability (3levels) |
Total |
| Low |
0-1 |
| Intermediate |
2-6 |
| High |
≥7 |
| Clinical probability (2levels) |
| Non-PE |
0-4 |
| PE |
>4 |
|
|
|
Treatment
Immediate treatment is required in acute PE after pulmonary
lobectomy cases. Before treatment, risk stratification is made
based on clinical manifestations, myocardial function and injury
markers for individualized treatment. PE was classified into high
risk and non-high risk groups by European Society of Cardiology (ESC). Notably, the non-high risk group is subdivided into
intermediate risk and low risk groups ( 1) ( Table 2). Hypoxia and hypocapnia are common in PE patients, among
whom most are with moderate hypoxia. Hypoxemia can be
corrected usually by oxygen inhalation through nasal catheter
but seldom mechanical ventilation. If mechanical ventilation is
required, particular attention are paid to avoid the side effects
of hemodynamics, especially in those with high risk PE because
mechanical ventilation-induced intrathoracic positive-pressure
may decrease venous reflux and progress RV failure (RVF). The
acute RVF and the RVF-induced low cardiac output are the
main cause for death. Trails show that RVF might be worsened
by the strong hyperolemic therapy through excessive stretching
of ventricular walls and/or reflex constriction suppression ( 31).
Appropriate liquid infusion may increase the low cardiac indexes
and cardiac indexes of PE patients with normal blood pressure
( 32). Thereby, the appropriate supportive treatment and
administration of vasoactive drugs are of great importance to PE
patients with RVF. Strong treatments are necessary for high risk patients,
including medication and mechanical thrombolysis. Notably,
thrombolysis treatment rapidly resolves thrombus embolism and
effectively improves the hemodynamic parameters; furthermore,
by thrombolysis treatment performed within 48 hours of the
presence of symptoms, the greatest benefit is achievable, and
it remains beneficial even to patients with symptoms for 6-14
days ( 33). immediate thrombolysis treatment is recommended
for severe PE patients who are definitely indicated with RVF by
echocardiography and thus can not undergo CT examination for
confirming diagnosis. Thrombolytic drugs such as streptokinase
(SK), urokinase (UK) and rtPA significantly increase the risk of
bleeding especially when there are bleeding-related relative risk
factors or complications. Since non-invasive imaging technique
has been successively used in diagnosing PE in the last decade,
the life-threatening bleeding is not common now. Therefore,
thrombolysis treatment is performed in all high risk PE patients
unless there are absolute contraindications. Pulmonay lobectomy
within 3 weeks is a contraindication for thrombolysis, however,
all contraindications are comparative since high risk PE patients
are encountering immediate life-threatening danger. Molina et al.
( 34) reported that a 9.6-day interval between the thrombolysis
drugs and surgery was relatively safe. Kameyama et al performed
thrombolysis treatment in high risk PE patients undergone
ineffective anticoagulant treatments, with thrombosis in lobes
or lung segments and hemodynamic instability after pulmonay
lobectomy ( 27). Notably, one case was performed 3.5 hours
later after surgery and all patients were successfully discharged
after active management. The r-tPA thrombolysis with a very
short plasma half-life was suggested in such patients because it
decreases bleeding. For patients with absolute contraindications of thrombolysis and unimproved hemodynamic status by thrombolysis,
embolectomy is the optimal treatment. Literatures suggested
immediate embolectomy after confirming of central PE ( 35).
At present, the indication of pulmonary arterial embolectomy
has spread to patients with RVF but without severe shock, with
the early mortality rate reported at 6-8% ( 36). If pulmonary
arterial embolectomy also fails to take effect immediately,
intraductal embolectomy or repulping surgery of thrombolysis
are considered. Non-high risk PE patients usually have favorable shortterm
prognosis. But the mixed data from 6 trials showed no
clinical benefits from thrombolysis treatment to this group of
patients ( 37), under this circumstance, anticoagulation therapy
should be performed immediately. The rapid anticoagulation
is performed only through parenteral anticoagulants such
as venous unfractionated heparin (UFH), subcutaneous
injection of low-molecular-weight heparin (LMWH) and
fondaparinux X a. For patients with high clinical PE probability,
anticoagulation therapy is given even before confirming
diagnosis ( 38). Moreover, parenteral anticoagulants usually need
to be administered together with the oral vitamin K antagonists
(VKAs). A meta analysis showed no significant difference in
recurrence rate of VTE, massive hemorrhage and mortality rate
between non-high risk PE patients treated with low-molecularweight
heparin and those with unfractionated heparin ( 39). For
PE patients with probability of massive hemorrhage or severe
renal failure, UFH is suggested as the initial anticoagulation
drug, and the partial thromboplastin time (PTT) needs to
be detected. Furthermore, the platelet value also needs to be
detected due to the risk of heparin-induced thrombocytopenia.
For most acute non-high risk PE patients without severe renal
failure, LMWH or fondaparinux is a preferred treatment and it’s
no need to monitor the dose of subcutaneous injection according
to the body weight. No matter UFH, LMWH or fondaparinux,
the anticoagulation treatment is supposed for taking at least
5 days. In addition, VKAs are to be used as early as possible
and had better combining with anticoagulation treatment.
The parenteral anticoagulants are stopped when international
normalized ratio (INR) staying at 2.0-3.0 for 2 days. Kilic ( 40)
suggested LMWH for intermediate risk groups so as to avoid
complications associated with thrombolytic therapy such as
bleeding and secondary pyothorax. Nevertheless, the second
urgent thrombolysis is usually required during thrombolytic
therapy for intermediate risk patients, though to whom the
optimal treatment is still controversial. A recent systematic review including 11 nonrandomized
studies demonstrated that proper outpatient follow-ups allowed
effective and safe home treatments in low risk patients. However,
it is controversial and the patients should be strictly selected.
There is still no literature definitely on whether thrombolytic
therapy is necessary for patients with PE in the single subsegmental lung on SCTPA. At present, thrombolytic therapy is performed
merely according to habits of doctors, clinical suspicion and
other examinations. Therefore, it is important to study in these
patients, whether the risk of fatal PE is greater than that of
anticoagulant complications ( 12). Vena cava filter can be placed into PE patients with
anticoagulant complications ( 42), but there hasn’t evidence
proving that the festbremsen brakes are beneficial to PE patients
at present.
|
|
|
| Table 2. Risk stratification according to expected pulmonary embolism-related early mortality rate. |
| Early mortality relative to PE |
Risk markers |
Potential treatment indications |
| |
Clinically shock or low blood pressure (b) |
RV dysfunction (c) |
Myocardial defects (d) |
| High >15% |
|
+ |
(+)a |
(+)a |
Thrombolysis or surgical embolectomy |
| Not high |
|
|
+ |
+ |
|
| Intermediate 3-15% |
— |
+ |
— |
Hospitalization |
| |
|
— |
+ |
|
| Low <1% |
— |
— |
— |
Early discharge or at-home therapy |
| Notes: a. In the presence of shock or hypotension it is not necessary to confirm RV dysfunction/injury to classify as high risk of PE-related early
mortality; b. Defined as a systolic blood pressure, less than 90 mmHg or a pressure drop of more than 40 mmHg for at least 15 min if not caused
by new-onset arrhythmia, hypovolaemia or sepsis; c. RV dilatation, hypokinesis or pressure overload on echocardiography; RV dilatation on spiral
computed tomography; brain natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP) elevation; right heart pressure elevation at right
heart catheterization; d. Positive cardiac troponin T or I . |
|
|
|
Preventions
Kalweit ( 8) reported that the mortality rate of PE was 92.6% in
patients with malignant tumor after pulmonary lobectomy hence
active prevention is advised, whereas there are still no established
prevention measures in thoracic surgery yet. The postoperative
prevention reported in western literatures include early
ambulation, intermittent sequential compression for improving
blood circulation in lower extremities, drug preventions (such
as UFH, LMWH and warfarin), subclavian vein intubation and
shortened time of mechanical ventilation, etc. Nevertheless,
these measures fail in completely preventing the occurrence
of PE. The clinical practice guideline of American College of
Chest Physicians (ACCP) ( 42) suggested the conventional use
of LMWH and fondaparinux in prevention of thrombus for
patients after thoracic surgery and appropriate use of mechanical
prevention such as progressive compression stockings and
intermittent inflation for postoperative patients with high risk
of hemorrhage. Due to the coagulation differences between the
yellow race and the white race, peroperative anticoagulation is
seldom applied in Japan, effect of which reported in Japan is not
as good as that in western studies ( 13). Nagahiro et al. ( 9, 43)
found that the efficacy of anticoagulation equals with that of
western studies only when performing intermittent pneumatic
compression (IPC) in preventing PE. Recurrence is potential in acute PE patients, the possibility
of which is 2%-10% in patients without VKAs persistent
anticoagulation therapy but less than 1% in treated cases ( 44). A
systematic review showed that more than 50% patients still had
residual thrombosis 6 months later ( 45). For PE patients, VKAs
treatment is advised successively for 3 months after pulmonary
lobectomy, and further VKAs treatment is decided according to
the individual conditions.
|
|
References
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur
Heart J 2008;29:2276-315.[LinkOut]
- Nikolaou K, Thieme S, Sommer W, Johnson T, Reiser MF. Diagnosing
pulmonary embolism: new computed tomography applications. J Thorac
Imaging 2010;25:151-60.[LinkOut]
- Auer RC, Schulman AR, Tuorto S, Gönen M, Gonsalves J, Schwartz L, et al.
Use of helical CT is associated with an increased incidence of postoperative
pulmonary emboli in cancer patients with no change in the number of fatal
pulmonary emboli. J Am Coll Surg 2009;208:871-8;discussion 878-80.[LinkOut]
- Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary
embolism and venous thrombosis by perioperative administration of
subcutaneous heparin. Overview of results of randomized trials in general,
orthopedic, and urologic surgery. N Engl J Med 1988;318:1162-73.[LinkOut]
- Ziomek S, Read RC, Tobler HG, Harrell JE Jr, Gocio JC, Fink LM, et al.
Thromboembolism in patients undergoing thoracotomy. Ann Thorac Surg
1993;56:223-6;discussion 227.[LinkOut]
- Dentali F, Malato A, Ageno W, Imperatori A, Cajozzo M, Rotolo N,
et al. Incidence of venous thromboembolism in patients undergoing
thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2008;135:705-6.[LinkOut]
- Sørensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous
thromboembolism and subsequent hospitalisation due to acute arterial
cardiovascular events: a 20-year cohort study. Lancet 2007;370:1773-9.[LinkOut]
- Kalweit G, Huwer H, Volkmer I, Petzold T, Gams E. Pulmonary embolism:
a frequent cause of acute fatality after lung resection. Eur J Cardiothorac
Surg 1996;10:242-6;discussion 246-7.[LinkOut]
- Nagahiro I, Watanuki Y, Sato S, Nakashima A. Venous velocity of the right
femoral vein decreases in the right lateral decubitus position compared to
the supine position: a cause of postoperative pulmonary embolism? Acta
Med Okayama 2007;61:57-61.[LinkOut]
- Kwek BH, Wittram C. Postpneumonectomy pulmonary artery stump
thrombosis: CT features and imaging follow-up. Radiology 2005;237:338-
41.[LinkOut]
- Işik F, Kara M, Tunçögür B, Sak SD, Kavukçu S. Significance of ligature
technique on the formation of pulmonary artery stump thrombosis in a
canine model. Acta Chir Belg 2005;105:203-6.[LinkOut]
- Sadigh G, Kelly AM, Cronin P. Challenges, controversies, and hot topics in
pulmonary embolism imaging. AJR Am J Roentgenol 2011;196:497-515.[LinkOut]
- Sakuragi T, Sakao Y, Furukawa K, Rikitake K, Ohtsubo S, Okazaki Y, et al.
Successful management of acute pulmonary embolism after surgery for
lung cancer. Eur J Cardiothorac Surg 2003;24:580-7.[LinkOut]
- Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med
2010;363:266-74.[LinkOut]
- Righini M, Le Gal G, Aujesky D, Roy PM, Sanchez O, Verschuren F, et al.
Diagnosis of pulmonary embolism by multidetector CT alone or combined
with venous ultrasonography of the leg: a randomised non-inferiority trial.
Lancet 2008;371:1343-52.[LinkOut]
- Stein PD, Sostman HD, Bounameaux H, Buller HR, Chenevert TL, Dalen
JE, et al. Challenges in the diagnosis of acute pulmonary embolism. Am J
Med 2008;121:565-71.[LinkOut]
- Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al.
Derivation of a simple clinical model to categorize patients probability of
pulmonary embolism: increasing the models utility with the SimpliRED
D-dimer. Thromb Haemost 2000;83:416-20.[LinkOut]
- Bruinstroop E, van de Ree MA, Huisman MV. The use of D-dimer
in specific clinical conditions: a narrative review. Eur J Intern Med
2009;20:441-6.[LinkOut]
- Carrier M, Righini M, Djurabi RK, Huisman MV, Perrier A, Wells PS, et al.
VIDAS D-dimer in combination with clinical pre-test probability to rule
out pulmonary embolism. A systematic review of management outcome
studies. Thromb Haemost 2009;101:886-92.[LinkOut]
- Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et
al. Multidetector computed tomography for acute pulmonary embolism. N
Engl J Med 2006;354:2317-27.[LinkOut]
- van Belle A, Büller HR, Huisman MV, Huisman PM, Kaasjager K,
Kamphuisen PW, et al. Effectiveness of managing suspected pulmonary
embolism using an algorithm combining clinical probability, D-dimer
testing, and computed tomography. JAMA 2006;295:172-9.[LinkOut]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of
radiation exposure. N Engl J Med 2007;357:2277-84.[LinkOut]
- Parker MS, Hui FK, Camacho MA, Chung JK, Broga DW, Sethi NN.
Female breast radiation exposure during CT pulmonary angiography. AJR
Am J Roentgenol 2005;185:1228-33.[LinkOut]
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute
pulmonary embolism: sensitivity and specificity of ventilation-perfusion
scintigraphy in PIOPED II study. Radiology 2008;246:941-6.[LinkOut]
- Anderson DR, Kahn SR, Rodger MA, Kovacs MJ, Morris T, Hirsch A,
et al. Computed tomographic pulmonary angiography vs ventilationperfusion
lung scanning in patients with suspected pulmonary embolism: a
randomized controlled trial. JAMA 2007;298:2743-53.[LinkOut]
- Pruszczyk P, Torbicki A, Pacho R, Chlebus M, Kuch-Wocial A, Pruszynski
B, et al. Noninvasive diagnosis of suspected severe pulmonary embolism:
transesophageal echocardiography vs spiral CT. Chest 1997;112:722-8.[LinkOut]
- Kameyama K, Huang CL, Liu D, Okamoto T, Hayashi E, Yamamoto Y, et
al. Pulmonary embolism after lung resection: diagnosis and treatment. Ann
Thorac Surg 2003;76:599-601.[LinkOut]
- Le Gal G, Righini M, Sanchez O, Roy PM, Baba-Ahmed M, Perrier A, et al.
A positive compression ultrasonography of the lower limb veins is highly
predictive of pulmonary embolism on computed tomography in suspected
patients. Thromb Haemost 2006;95:963-6.[LinkOut]
- Stein PD, Chenevert TL, Fowler SE, Goodman LR, Gottschalk A, Hales
CA, et al. Gadolinium-enhanced magnetic resonance angiography for
pulmonary embolism: a multicenter prospective study (PIOPED III). Ann
Intern Med 2010;152:434-43,W142-433.
- Wolfe MW, Skibo LK, Goldhaber SZ. Pulmonary embolic disease:
diagnosis, pathophysiologic aspects, and treatment with thrombolytic
therapy. Curr Probl Cardiol 1993;18:587-633.[LinkOut]
- Ghignone M, Girling L, Prewitt RM. Volume expansion versus
norepinephrine in treatment of a low cardiac output complicating an acute
increase in right ventricular afterload in dogs. Anesthesiology 1984;60:132-5.[LinkOut]
- Mercat A, Diehl JL, Meyer G, Teboul JL, Sors H. Hemodynamic effects
of fluid loading in acute massive pulmonary embolism. Crit Care Med
1999;27:540-4.[LinkOut]
- Daniels LB, Parker JA, Patel SR, Grodstein F, Goldhaber SZ. Relation of
duration of symptoms with response to thrombolytic therapy in pulmonary
embolism. Am J Cardiol 1997;80:184-8.[LinkOut]
- Molina JE, Hunter DW, Yedlicka JW, Cerra FB. Thromboly tic
therapy for postoperat ive pulmonary embolism. Am J Surg
1992;163:375-80;discussion 380-1.[LinkOut]
- Chen Q, Tang AT, Tsang GM. Acute pulmonary thromboembolism
complicating pneumonectomy: successful operative management. Eur J
Cardiothorac Surg 2001;19:223-5.[LinkOut]
- Meneveau N, Séronde MF, Blonde MC, Legalery P, Didier-Petit K, Briand
F, et al. Management of unsuccessful thrombolysis in acute massive
pulmonary embolism. Chest 2006;129:1043-50.[LinkOut]
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared
with heparin for the initial treatment of pulmonary embolism: a metaanalysis
of the randomized controlled trials. Circulation 2004;110:744-9.[LinkOut]
- Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler
TI. Early anticoagulation is associated with reduced mortality for acute
pulmonary embolism. Chest 2010;137:1382-90.[LinkOut]
- Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin
compared with intravenous unfractionated heparin for treatment of
pulmonary embolism: a meta-analysis of randomized, controlled trials.
Ann Intern Med 2004;140:175-83.[LinkOut]
- Kilic D, Akin S, Findikcioglu A, Bilen A, Aribogan A, Hatipoglu A.
Low-molecular-weight heparin for treatment of submassive pulmonary
embolism after pneumonectomy. Gen Thorac Cardiovasc Surg
2007;55:287-9.[LinkOut]
- Squizzato A, Galli M, Dentali F, Ageno W. Outpatient treatment and early
discharge of symptomatic pulmonary embolism: a systematic review. Eur
Respir J 2009;33:1148-55.[LinkOut]
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, et
al. Antithrombotic therapy for venous thromboembolic disease: American
College of Chest Physicians Evidence-Based Clinical Practice Guidelines
(8th Edition). Chest 2008;133:454S-545S.[LinkOut]
- Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Intermittent
pneumatic compression is effective in preventing symptomatic pulmonary
embolism after thoracic surgery. Surg Today 2004;34:6-10.[LinkOut]
- Agnelli G, Prandoni P, Becattini C, Silingardi M, Taliani MR, Miccio M, et
al. Extended oral anticoagulant therapy after a first episode of pulmonary
embolism. Ann Intern Med 2003;139:19-25.[LinkOut]
- Nijkeuter M, Hovens MM, Davidson BL, Huisman MV. Resolution of
thromboemboli in patients with acute pulmonary embolism: a systematic
review. Chest 2006;129:192-7.[LinkOut]
Cite this article as: Wang ZF, Pei C, Ma LC, Wang DY, Zhou JF,
Wang W, Shen JF, Xu ZQ, He JX. Acute pulmonary embolism after
pulmonary lobectomy. J Thorac Dis 2012;4(1):76-82. doi: 10.3978/
j.issn.2072-1439.2011.10.02
|