Retreatment with pemetrexed chemotherapy in advanced non-small cell lung cancer patient
Introduction
Pemetrexed is a pyrrolopyrimidine antifolate which inhibits thymidylate synthase, glycinamide ribonucleotide formyltransferase, and ihydrofolate reductase. Pemetrexed was approved by the FDA in 2004 for the treatment of patients with malignant pleural mesothelioma (1) and approved as second-line (2), first-line (3) and maintenance treatment (4,5) in advanced non-small cell lung cancer (NSCLC) now.
Pemetrexed has shown to be less toxic than other chemotherapy regimens, with excellent efficacy and safety in non-squamous NSCLC. There is no guideline for chemotherapy in NSCLC patients who failed second-line/third-line treatment. However, many patients have actually received further-line chemotherapy in the real-world setting. Some studies have conducted trials to evaluate the efficacy of pemetrexed retreatment in patients with malignant pleural mesothelioma (6,7), however, no study investigated the efficacy of pemetrexed retreatment in NSCLC.
In the present study, we investigated the efficacy of retreatment of pemetrexed after failure of prior pemetrexed-based treatment in advanced NSCLC, and to explore which patients may benefit from retreatment.
Patients and methods
Patient eligibility
Twenty-five consecutive, unselected NSCLC patients, who were admitted to Zhejiang Cancer Hospital from Dec 2009 to Dec 2012, were included in our study. NSCLC staging was performed for all the patients according to the 7th TNM classification. Inclusion criteria were as follows: (I) Pathologically proven non-squamous NSCLC; (II) the disease recurrence was confirmed using chest computed tomography (CT), brain MRI and bone scan as well as ultrasound examination and/or CT of the abdomen; (III) without any local treatment like radiotherapy or interventional therapy during the period of pemetrexed therapy; (IV) at least one measurable lesion and an Eastern Cooperative Oncology Group performance status (PS) of 0 to 2.
Treatment
All patients were given pemetrexed 500 mg/m2 as a 10-min intravenous infusion on day 1 every 21 days. Dexamethasone (4.5 mg) was taken twice daily on the day before, the day of, and the day after each dose of pemetrexed. Folic acid supplementation and vitamin B12 were taken beginning one week prior to the first dose of pemetrexed and continued until one month after treatment discontinuation.
Response evaluation
All patients were followed up every 6 weeks with imaging examination during treatment with pemetrexed. Objective tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Disease control rate (DCR) was defined as the addition of objective response and stabilization.
Toxicity evaluation
The toxicity profile of pemetrexed was assessed by reviewing medical records including 25 patients. Severity of adverse reactions was determined based on the requirements of dosage reduction or discontinuation of pemetrexed. All such toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 3.0 (CTC3.0).
Follow-up
All the patients were to be evaluated for tumor response and PFS. Follow-up rate was 100%. The last follow-up date was July 31, 2013.
Statistical analysis
PFS encompassed the time from the first cycle of therapy to documented progression or death from any cause, or until the date of the last follow-up visit for patients who were still alive and who had not progressed. Survival analysis was conducted with a Kaplan-Meier analysis and log-rank test. All statistical tests were analyzed using the computer software SPSS version 17.0 (SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
A total of 25 patients were included in the study. There were 16 males and 9 females. All of the 25 patients were with advanced stage. Performance score 0-1 was present in 10 patients (40%) and PS 2 accounted for 60%. The median age of the patients was 59.5 years (range, 38-74 years). Twenty-four patients were with adenocarcinoma and one with large cell lung cancer. All of the 25 cases underwent cytotoxic chemotherapy or targeted treatment between the initial and retreatment pemetrexed therapy. Twenty-two patients received EGFR-TKI therapy before pemetrexd retreatment in our study. Among the 22 patients, 11 patients received EGFR-TKI treatment in second-line, 9 in third-line and 2 in fourth-line. Among the 13 patients with initial pemetrexed PFS >6 months, 10 patients treated with EGFR-TKI before pemetrexd retreatment. Patients’ characteristics are shown in Table 1.
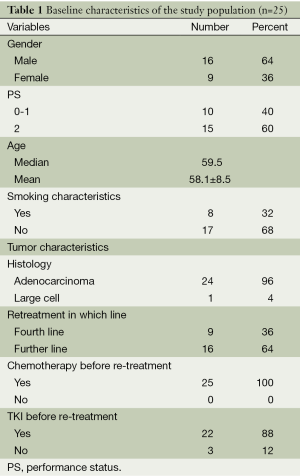
Full table
Response data and survival analysis
Twelve patients achieved a PR and thirteen with SD in the initial pemetrexed treatment, accounting for a DCR of 100%. There were one patient with a PR to the retreatment, while 9 patients had SD and 15 patients had PD. Median PFS during initial pemetrexed-based treatment was 6.6 months (95% CI: 5.0-15.5 months), but 1.5 months during pemetrexed retreatment (95% CI: 0.8-2.4). Thirteen patients had PFS more than six months (>6 months) in the initial pemetrexed treatment and 12 with PFS less than six months (≤6 months). The median survival time for all patients was 21.5 months. The median OS from the beginning of the 2nd pemetrexed was 7.1 months (95% CI: 5.5-10.7 months). Among the 22 patients treatment with EGFR-TKI, 6 tested the EGFR mutation, 2 with EGFR mutation and 4 with wild-type. The PFS of pemetrexed retreatment for the 2 patients with EGFR mutation was 1.2 and 1.0 months for the patients with EGFR wild-type (P=0.14).
The relationship between initial treatment and retreatment efficacy
The overall DCR in the retreatment group was 40%. The retreatment DCR was 46.2% (6/13) in patients who had PFS duration more than 6 months (>6 months) in the prior pemetrexed and 33.3% (4/12) in the patients who had PFS duration less than 6 months (≤6 months), and the PFS was 2.2 and 1.1 months in the two group, respectively, (P=0.036) (Figure 1).
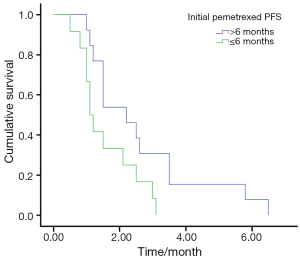
Factors affecting PFS by univariate and multivariate analysis
Results of univariate analysis for PFS of retreatment are shown in Table 2. The PS score (P=0.031) and initial pemetrexed PFS (P=0.036) were the factors influence the PFS of retreatment pemetrexed (Table 2).
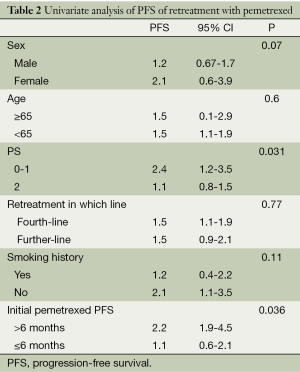
Full table
A multivariate Cox regression model was constructed with the incorporation of age, sex, PS, smoking history and initial pemetrexed PFS. PS remained as independent prognostic factor for PFS of retreatment pemetrexed (P=0.045).
Toxicities of treatment
Toxicity was evaluated in all the patients. The most common adverse event was hematological toxicities in 16 patients (64.0%), including 5 patients with grade 3-4. Two patients demonstrated fatigue after being retreated with pemetrexed therapy (grade 3). Three dosage reduction were occurred.
Discussion
To the best of our knowledge, our represents the first data to assess whether pemetrexed retreatment confers any clinical benefit in patients with advanced NSCLC. In our series, we obtained a DCR of 40% with a median duration of this control of 1.5 months.
It was investigated that second-line therapy was given to 40-60% of patients and 20-30% of patients could receive third-line therapy or further therapy recently (8,9). The availability of new chemotherapy regimens like pemetrexed with low toxicity agents increases the chance for the advanced NSCLC patients to receive further treatment. However, with the decreased of PS and less tolerance of toxicity, few treatment choice was explored for the patients who received further-line treatment.
Two large randomized phase III studies—the JMDB (3) and JMEI (2) trials showed a statistically significant efficacy in patients with non-squamous histology with pemetrexed-based treatment in NSCLC. Another two randomized phase III studies—the JMEN (4) and PARAMOUNT (5) trials also showed that pemetrexed had a well efficacy and less toxicity as maintenance treatment in NSCLC patients.
Retreatment with pemetrexed was reported as effective as second-line therapy in malignant pleural mesothelioma patients who achieved a durable (>12 months) disease control with first-line pemetrexed by Ceresoli et al. and Bearz et al. studies (6,7). In our study, the DCR and ORR were 40% and 4%, which showed a similar efficacy as second-line treatment.
A limitation of this study may be the retrospective design and small number of patients. However, with no cases in previous clinical studies, our retrospective study can also be considered to be meaningful.
In conclusion, our results indicated that retreatment of pemetrexed could be consider as one of treatment option for the patients with PFS more than six months in the initial pemetrexed-based chemotherapy in NSCLC. Further prospective evaluation of this therapeutic option is warranted.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hazarika M, White RM, Johnson JR, et al. FDA drug approval summaries: pemetrexed (Alimta). Oncologist 2004;9:482-8. [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [PubMed]
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [PubMed]
- Ceresoli GL, Zucali PA, De Vincenzo F, et al. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer 2011;72:73-7. [PubMed]
- Bearz A, Talamini R, Rossoni G, et al. Re-challenge with pemetrexed in advanced mesothelioma: a multi-institutional experience. BMC Res Notes 2012;5:482. [PubMed]
- Song Z, Yu Y, Chen Z, et al. Third-line therapy for advanced non-small-cell lung cancer patients: feasible drugs for feasible patients. Med Oncol 2011;28 Suppl 1:S605-12. [PubMed]
- Girard N, Jacoulet P, Gainet M, et al. Third-line chemotherapy in advanced non-small cell lung cancer: identifying the candidates for routine practice. J Thorac Oncol 2009;4:1544-9. [PubMed]


