Image-guided bronchoscopy for histopathologic diagnosis of pure ground glass opacity: a case report
Introduction
Particular interest in peripheral pulmonary lesions (PPLs) and ground glass opacities (GGOs) of the lung has grown in recent years. If malignancy is suspected, the strategies for evaluation are positron emission tomography scan, non-surgical biopsy, or surgical resection (1). For non-surgical procedures, guidelines recommend extensive tissue sampling for accurate histopathologic and molecular diagnosis (2,3). Previous studies on transthoracic needle aspiration (TTNA) have reported that a higher percentage of GGO component contributed to a lower diagnostic yield for these types of PPLs (4,5).
Bronchoscopy is another non-surgical option for diagnosis of peripheral lung cancer (1). Endobronchial ultrasound with a guide sheath (EBUS-GS) had been reported to increase the diagnostic yield of transbronchial biopsy (TBB) (6,7) and the addition of virtual bronchoscopic navigation (VBN) can improve this yield further (8). Majority of studies on TBB for PPLs included solid lesions but reports on how to improve the procedure for GGOs are lacking. In this report, we present the case of a patient with pure GGO of the lung that was successfully diagnosed by TBB.
Case presentation
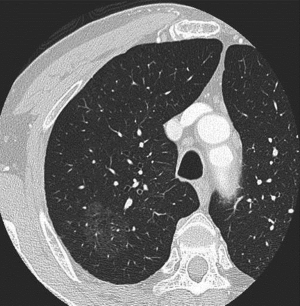
A 69-year-old male was referred to our department because of an incidental chest computed tomography (CT) scan finding of a pure GGO PPL, measuring 38 mm in the largest diameter, and located in the right segment 2 (Figure 1). He was asymptomatic, a 50-pack year tobacco smoker, and without history of malignancy.
This study was approved by the Institutional Review Board of the hospital and informed consent was obtained from the patient.
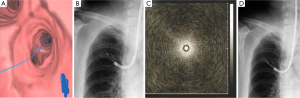
Bronchoscopy was performed under local anesthesia with conscious sedation using BF 1T260 (outer diameter of 5.9 mm, working channel diameter of 2.8 mm; Olympus, Tokyo, Japan). VBN (LungPoint; Broncus Technologies, Inc., Mountain View, CA, USA) was used to plan the route to the target lesion (Figure 2A). Radial EBUS probe (UM-S20-20R, Olympus, Tokyo, Japan) with a guide sheath (2.6 mm compatible channel diameter, 2.55 mm maximum outer diameter; GS Kit K-203, Olympus, Tokyo, Japan) was inserted into the affected bronchus under fluoroscopy guidance (Figure 2B). The EBUS image showed blizzard sign (9) (Figure 2C). Cytology specimen was collected by brush (length 10 mm, diameter 2.0 mm); standard biopsy forceps with a 1.9 mm maximum outer diameter was used to collect a total of five histology samples (Figure 2D). There were no complications after bronchoscopy.
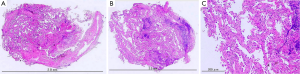
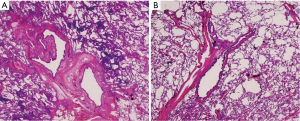
Cytology examination was negative for malignant cells. The consecutive TBB specimens were examined histopathologically in the order that they were collected. The first and second specimens were negative for malignant cells, the third showed a few atypical cells, and the fourth and fifth specimens showed adenocarcinoma with lepidic growth pattern (Figure 3). After right upper lobectomy, the definitive diagnosis was confirmed to be minimally invasive adenocarcinoma (Figure 4) pstage IB (T2aN0M0). No further adjuvant therapy was administered.
Discussion
The introduction of chest CT scan screening has led to an increased interest in PPLs, particularly GGOs, which can be pre-invasive lesions in the lung adenocarcinoma growth spectrum. Non-surgical options for pathology diagnosis are by bronchoscopic or transthoracic approach. Studies on TTNA of GGOs demonstrated that obtaining core tissue resulted to high diagnostic yields and that pure GGOs were more difficult to diagnose (4,5,10). On the other hand, more studies on bronchoscopy as a diagnostic tool for GGOs are lacking. One study reported that EBUS-GS TBB of GGO-dominant lesions had a diagnostic yield of less than 60 percent (11). Further refinements that focus on tissue procurement from GGO-predominant or pure GGO PPLs are needed.
For EBUS-GS transbronchial sampling, it has been reported that an EBUS probe within a lesion and VBN were useful for tumor localization prior to biopsy, leading to improved diagnostic yields (6,8). We previously reported blizzard sign as a specific EBUS image for GGO (9). For this particular case, this sign helped us locate the biopsy site after it was detected by EBUS within the bronchial subsegment that was specified by VBN. At present, guide sheath kits (forceps, brush, and guide sheath) come in two sizes, large and small. Generally, bronchoscopists choose one of them according to PPL characteristics and physician preferences. For this patient, we chose to use the large size for the purpose of obtaining extensive amounts of tissue.
Histologically, GGOs are described to have replacing growth patterns along thickened but preserved alveolar walls; this lepidic growth usually corresponds to the ground glass component (3). It was recommended that high quality tissue samples were more effective than cytology samples for diagnosis of GGOs (2,3). To our knowledge, there have been no reports yet describing the optimal number of TBB specimens required to diagnose GGOs. In this case, cytology samples were negative and only the TBB samples were diagnostic. Specifically, the structural integrity of adenocarcinoma with lepidic growth pattern was demonstrated in the latter (fourth and fifth) TBB specimens. Correlating this with the histopathologic findings of thickened bronchial wall in the resected specimen (Figure 4), we considered that obtaining around five histology samples by a larger device would be required to penetrate the thick smooth muscle layer.
In conclusion, image-guided bronchoscopy and use of a larger sampling device during TBB may be helpful for histopathologic diagnosis of pure GGO.
Acknowledgements
We are grateful to Dr. Junko Watanabe, Dr. Ikkoh Yasuda, and Dr. Keisuke Kirita for their kind assistance in making this report.
Funding: This work was supported by the National Cancer Center Research and Development Fund (25-A-12).
Disclosure: The authors declare no conflict of interest.
References
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:668-84. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Kim TJ, Lee JH, Lee CT, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2008;190:234-9. [PubMed]
- Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [PubMed]
- Tamiya M, Okamoto N, Sasada S, et al. Diagnostic yield of combined bronchoscopy and endobronchial ultrasonography, under LungPoint guidance for small peripheral pulmonary lesions. Respirology 2013;18:834-9. [PubMed]
- Sasada S, Izumo T, Chavez C, et al. Blizzard sign as a specific endobronchial ultrasound image for ground glass opacity: a case report. Respiratory Medicine Case Reports 2014;12:19-21.
- Lu CH, Hsiao CH, Chang YC, et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol 2012;7:143-50. [PubMed]
- Izumo T, Sasada S, Chavez C, et al. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J Thorac Dis 2013;5:745-50. [PubMed]


