Pulmonary hypertension in end-stage renal disease and post renal transplantation patients
Introduction
Pulmonary hypertension (PH) has emerged as a major complication of several systemic disorders. The estimated prevalence rates of PH range from 16-58% for patients receiving hemodialysis (HD), 12-42% for patients receiving peritoneal dialysis (PD), and 5-14% among patients who have undergone renal transplantation (1-11). Importantly, it has been found that patients with PH who experience renal failure show significantly higher rates of morbidity and mortality (1,2). The pathogenesis of renal failure-associated PH is complex, and it may include metabolic and hormonal derangements, high cardiac output due to arterio-venous fistula (AVF), impaired endothelial function, anemia, fluid overload, and other factors (3,12,13). Because of the plethora of possible mechanisms by which it exerts its effects, PH related to renal failure falls into group 5 (14).
Impaired lung function and exercise capacity are commonly observed in patients with chronic renal failure. Such limitations are due to several factors, including inflammation, myopathy, neuropathy, metabolic acidosis, and others (15-18). However, there is little information available regarding pulmonary function parameters and functional capacity among patients with renal failure-associated PH.
In this study, we sought to determine the incidence of PH in three groups: patients receiving HD, patients receiving PD, and patients who had undergone renal transplantation. Additionally, we sought to compare the results of the pulmonary function tests (PFTs) and the six-minute walk test (6MWT) in the PH patients versus those without PH in the three groups.
Materials and methods
Study population
Patient selection
This was a prospective, descriptive clinical study conducted between November 2008 and June 2010. Patients were included in the study if they were aged 18 years or above, were receiving HD or PD or were post-renal transplantation patients, and for whom the cause of PH was unknown. Patients were excluded they were known to have a chest wall deformity, parenchymal lung disease, a history of pulmonary embolism, collagen vascular disease, congestive heart failure, significant valvular heart disease, or chronic liver disease. Additionally, six patients were not included in the study because they were unable to perform the PFTs and 6MWT. In addition, 12 patients refused to participate in the study.
Patient assessment
A standard form was used to collect information regarding demographics, clinical factors, duration of dialysis, etiology of renal failure, and vascular access location. Echocardiography, PFTs, arterial blood gas (ABG) sampling, 6MWT, and blood testing for hemoglobin, creatinine, albumin, serum calcium, phosphorus, and parathyroid hormone levels were performed in all participants. For patients with PH, chest radiography and computed tomography were included in the systemic diagnostic evaluation.
Echocardiograms and estimation of systolic pulmonary artery pressure (SPAP)
Two-dimensional, M-mode, and Doppler echocardiography exams were performed on all of the participants by two experienced echo technicians dedicated to this study. The Phillips Sonos 5,500 imaging system (Phillips Co., Andover, MA, USA), equipped with a 3.2 MHz transducer, was used. Multiple views using different acoustic windows were obtained to measure the most optimal tricuspid regurgitation (TR) jet signal using continuous wave (CW) Doppler at a sweep speed of 100 to 200 mm/s. Only CW signals that demonstrated the peak velocity of the TR jet were used for this analysis. SPAP was estimated based on the modified Bernoulli equation as follows (19): 4 V2 (V = peak velocity of TR in meters per second, obtained using the CW Doppler) was added to the estimated right atrial pressure (RAP). The RAP was estimated based on the dimensions of the inferior vena cava (IVC) during inspiration. The RAP was estimated to be 5 mmHg if the IVC size was less than 2.0 cm and collapsed by 50% during inspiration, 10 mmHg if the IVC was less than 2.0 cm and did not collapse by 50%, 15 mmHg if the IVC was greater than or equal to 2.0 cm and collapsed more than 50%, and 20 mmHg if the IVC was greater than or equal to 2.0 cm and did not collapse by 50%. A patient was considered to have PH if the SPAP was greater than or equal to 40 mmHg (20). All of the studies were evaluated off line by an experienced echocardiographer who was blinded to the patients’ clinical data.
Physiological measurements
Immediately after echocardiography, the patients performed PFTs (PFT Masterscreen; Jaeger, Hoechberg, Germany) using standard methodologies. These tests included spirometry, plethysmography, and measurement of the diffusion capacity of the lung for carbon monoxide (DLco) (21-23). ABG values (Rapid Lab 865; Bayer, Plymouth, UK) were obtained for the partial pressure of oxygen (PaO2), the partial pressure of carbon dioxide (PaCO2), and the extent of oxygen saturation (SaO2). After the PFTs and ABG sampling, the patients were asked to perform the 6MWT in accordance with ATS guidelines (24). SpO2 and the Borg dyspnea index (25) were recorded at the beginning and end of a six-minute walk. At the end of the test, the total distance walked in meters was documented.
In the HD group, echocardiograms, PFTs, and the 6MWT were performed within one hour after the completion of HD to assure that the patients were at the optimal dry weight. In the PD group, the dialysis fluid was drained before the echocardiograms and physiological tests were performed.
Blood samples
Blood tests for hemoglobin, creatinine, albumin, serum calcium, phosphorus, and parathyroid hormone level were performed in all participants within one week of the physiological studies.
Statistical analysis
Descriptive statistics (the mean values, standard deviations, and percentages) were used to describe the quantitative and categorical study variables. Chi-square statistics were used to assess the differences between proportions. Student’s t-test for independent samples was applied to compare the mean values of continuous variables. A two-sided P value <0.05 was considered statistically significant. All of the analyses were performed using Statistical Package for the Social Sciences software (SPSS, version 18.0; SPSS Inc., Chicago, IL, USA).
Results
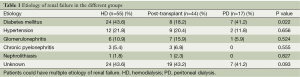
A total of 116 patients (HD =55, post renal transplantation =44, and PD =17) were eligible for the study. Chronic renal failure secondary to diabetes mellitus was more frequently noted in the HD and PD groups than in the post renal transplantation group (Table 1) (P=0.022). However, the distributions of other causes of chronic renal failure were not significantly different among the three groups (Table 1) (all P>0.05).
Full table
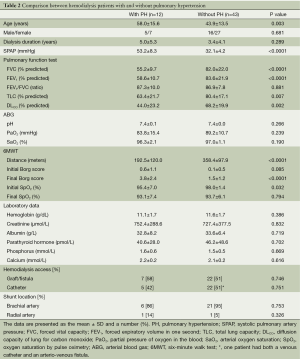
In the HD group, PH was detected in 12 (21.8%) patients. The comparisons of the demographic and clinical characteristics and the laboratory data for the HD patients with PH and those without PH are shown in Table 2. The HD patients with PH were significantly older than those without PH (P=0.003). No differences in gender or the duration of dialysis were noted between the patients with and without PH. However, the PH group had significantly lower forced vital capacity (FVC) [(55.2±9.7)% predicted vs. (82.0±22.0)% predicted, P<0.0001], forced expiratory volume in one second (FEV1) [(58.6±10.7)% predicted vs. (83.6±21.9)% predicted, P<0.0001], total lung capacity (TLC) [(63.4±21.7)% predicted vs. (80.4±17.1)% predicted, P=0.007], and DLCO [(44.0±23.2)% predicted vs. (68.2±19.9)% predicted, P=0.002] compared with the patients without PH. In addition, the HD patients with PH had a significantly shorter walking distance [(192.5±120.0) vs. (358.4±97.9) meters, P<0.0001] and a higher dyspnea score at the end of the 6MWT [(3.8±2.4) vs. (1.5±1.2), P<0.0001] compared with the patients without PH. There was no significant difference between those with and without PH with regard to hemoglobin, creatinine, albumin, phosphorus, calcium, parathyroid hormone levels, HD access, and shunt location.
Full table
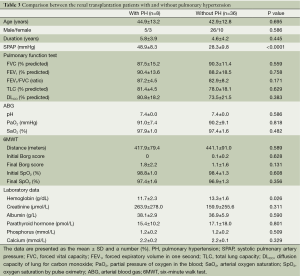
In the post renal transplantation group, PH was noted in eight (18.2%) patients. The clinical characteristics and laboratory data for the patients with and without PH are shown in Table 3. No between-group differences were observed with regard to age, gender, disease duration, PFTs, ABG, or 6MWT. With regard to the laboratory results, the hemoglobin level was the only parameter that was significantly lower in the PH group [(11.7±2.3) vs. (13.3±1.6) g/dL, P=0.026].
Full table
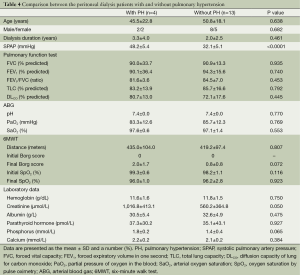
Among the patients receiving PD, PH was noted in four (23.5%) patients. However, in this group, the clinical characteristics, physiological parameters, and laboratory data did not differ between patients with and without PH (Table 4).
Full table
The clinical characteristics, physiological parameters, and laboratory data for the patients with PH in all three groups (HD, post renal transplantation, and PD) are summarized in Table 5.
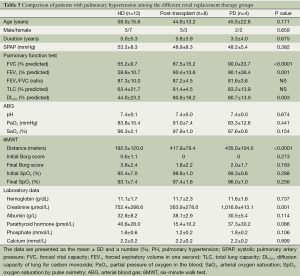
Full table
During the study period, three patients with PH died (one each in the HD, PD, and post renal transplantation groups) and five patients without PH died (two each in the HD and PD groups and one in the post renal transplantation group). A survival analysis was not performed due to the small sample size and the low number of deaths in each group.
Discussion
The present study demonstrates that PH was relatively common in the patients receiving HD and PD and the post renal transplantation patients. However, significantly impaired lung function and functional capacity were only noted in the patients with PH receiving long-term HD.
The prevalence of PH among the patients receiving long-term HD ranges from 16-58%, depending on the definition of PH, the methodology, the ethnicity of the patients, the institution, and the region of the world (1-7). For example, the majority of the cited studies define PH as an SPAP ≥35 mmHg. However, when the cut-off value of 45 mmHg was applied, the PH estimate was substantially lower (16-20%) (1,2). Furthermore, a higher prevalence of PH was noted when the echocardiograms were performed before dialysis (48-58%) (1,3) compared to when the echocardiograms were performed immediately after dialysis (29-39%) (2,5,6). Many factors have been suggested to contribute to the development of PH in end-stage renal disease. For example, the increase in cardiac output in response to AVF among the patients receiving HD has been implicated in the pathogenesis of PH (5,7,26). However, the lack of a significant difference in cardiac output between the patients with and without PH (27) and the reduction in cardiac output and PAP among the HD patients who underwent kidney transplantation regardless of the status of AVF (whether it remained open or closed) (7) suggest that other mechanisms are involved in the development of PH.
In their study, Ramasubbu et al. (1) reported that 63% of HD patients with PH exhibited echocardiographic evidence of elevated pulmonary capillary wedge pressure (PCWP). In addition, they noted a significant correlation between PAP and PCWP. In another study, significant increases in the cardiac index, the IVC diameter, and the left atrial diameter, which are all markers of volume overload, were noted in the PH patients receiving long-term HD (2). Collectively, these studies illustrate that chronic volume overload may play a role in the pathogenesis of PH. Other risk factors for PH have also been described, including age, the duration of chronic renal failure, hyperparathyroidism, and increased pulmonary vascular stiffness secondary to endothelial dysfunction (4,7,28,29). Although the purpose of the present study was not to identify variables associated with an increased risk of PH, we found no significant difference between the HD patients with and without PH in terms of age, dialysis duration, hemoglobin, serum calcium, phosphorus, or parathyroid hormone level. Furthermore, neither the type of HD access nor the shunt location was associated with PH. Interestingly, we noted that the PFT parameters and 6MWT were markedly reduced in the PH patients receiving long-term HD compared with those without PH. To the best of our knowledge, this is the first study to examine the effect of PH on lung function indices and functional capacity among patients with end-stage renal disease.
Because uremic patients often experience dysfunctions in multiple systems, aberrations in PFTs are not uncommon. Previous studies that have examined the effect of dialysis on PFT parameters revealed that a restrictive ventilatory defect was commonly observed among HD patients (16,30,31). In agreement with the cited studies, we noted a similar finding among the HD patients. However, in the present study, marked impairments in lung volume and DLco were also noted in the HD patients with PH, suggesting that this group may represent a distinct entity. Bush and Gabriel (31) and Herrero et al. (32) have suggested that the reduction in DLco may be due to chronic pulmonary fibrosis. However, the findings in the present study do not support this hypothesis because our patients with PH demonstrated no evidence of pulmonary fibrosis as assessed by HRCT. In addition, the PFT parameters of the PD and post-transplantation patients were within the normal range, regardless of whether PH was present or not, implying that a separate mechanism was involved in the pathogenesis of PH among HD patients.
In support of this notion, a previous study showed significant impairment in nitric oxide production, a marker of endothelial dysfunction, among PH patients receiving HD (7). Vascular endothelial growth factor (VEGF) is a glycoprotein with potent angiogenic and vascular permeability-enhancing properties, and it is involved in one of the important pathways that has been implicated in the pathogenesis of PH (33,34). Interestingly, hypoxia and acidosis, either alone or in combination, are frequently encountered in dialysis patients, and both conditions are potent inducers of VEGF expression (35-37). Recently, Yuan et al. (38) showed that a high serum level of VEGF was an independent predictor of mortality in dialysis patients. However, in the cited study, it was not clear whether the increase in the serum level of VEGF was associated with the presence of PH. As such, future studies are needed to determine the role of endothelial dysfunction and VEGF in the development of PH among dialysis patients.
Several factors, including respiratory status, cardiac involvement, skeletal muscle weakness, malnutrition, metabolic acidosis, corticosteroids, and others, lead to exercise intolerance, which manifests as reduced walking distance among chronic renal failure patients (15,39,40). A striking finding of our study was the significantly shorter walking distance among the PH patients receiving HD compared with those without PH. Moreover, the walking distance was significantly shorter in the HD patients with PH compared with those with PH receiving PD and post renal transplantation patients, substantiating the idea that the presence of PH in HD patients is distinct and has a deleterious effect on the functional capacity of these patients. Because the 6MWT is simple, inexpensive, reproducible, and well-received by patients because it mimics the effort required for daily physical activity, it has become a popular tool for predicting the prognoses of patients with various pulmonary and non-pulmonary diseases and is used as a surrogate marker for responsiveness to therapy in many clinical drug trial studies. Surprisingly, very few studies have attempted to characterize the effect of dialysis on the 6MWT. In addition, there is no information on the best time to perform the 6MWT among dialysis patients. Although in the present study, the walking test was conducted within one hour of the HD to ensure that the patients were at the optimal dry weight, this timing may have had a negative impact on the walking test.
Previous studies (41,42) noted that the patients receiving HD had a significant increase in whole body and muscle protein breakdown, along with a significant increase in inflammatory markers, including interleukin (IL)-6 and fibrinogen, during HD and for two hours afterward. This result implies that HD induces an acute inflammatory response in addition to the persistent chronic inflammatory state that occurs in end-stage renal disease patients. Nonetheless, the significantly shorter walking distance noted in the current study among patients with PH who were receiving HD suggests that PH has a significant negative impact on functional capacity. Thus, future studies are warranted to explore the value of the 6MWT as a screening tool to identify patients with PH and to determine whether this test can be used to predict mortality among PH patients receiving HD.
In the current study, PH was detected in four (23.5%) patients receiving PD. The reported prevalence of PH in PD patients ranges from 12-42%, mostly because of variation in the patient selection criteria (3,8,9). However, because of the small number of patients with PH noted in the present study and the lack of significant differences in the PFT parameters and the 6MWT results between those with and without PH, it is difficult to draw a firm conclusion. Large-scale studies are needed to explore the true impact of PH on the physiological parameters among patients receiving PD.
Renal transplantation is regarded as the gold standard to restore renal function among end-stage renal disease patients. Simmons et al. (43) reported that pro-inflammatory cytokines and oxidative stress markers return to a normal baseline level that is similar to that of healthy controls within two months of renal transplantation. The use of immunosuppressive medications, the restoration of renal function, or perhaps the combination of both may account for the normalization of the markers of oxidative stress and pro-inflammatory cytokines. This may explain the significant reduction in PAP and the normalization of pulmonary function parameters reported in previous studies of patients who underwent renal transplantation (7,12,13,16,44). In the present study, PH was noted in 18% of the patients in the renal transplant group. However, we found no significant difference in the PFT parameters or in the walking distance between those with or without PH. As such, longitudinal follow-up is needed to determine the clinical significance of detecting PH among renal transplant patients.
In conclusion, in this study, we show that PH is commonly observed among patients with end-stage renal disease and post renal transplantation patients. However, the PFT and 6MWT results were only severely compromised in the patients with HD-associated PH. Whether the aberrations in the pulmonary function parameters and functional capacity results that were observed in the HD patients in this study can potentially be used to predict the presence of PH or perhaps as a marker of disease severity to expedite kidney transplantation is unknown and should be explored in future studies.
Acknowledgements
The study was approved by the Institutional Review Board/Ethics Committee of the College of Medicine, King Saud University, Riyadh, Saudi Arabia, and written informed consent was obtained from all the study participants.
This work was supported by a grant from King Saud University, Deanship of Scientific Research, College of Medicine Research Center, Riyadh, Saudi Arabia.
Disclosure: The authors declare no conflict of interest.
References
- Ramasubbu K, Deswal A, Herdejurgen C, et al. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: prevalence and clinical significance. Int J Gen Med 2010;3:279-86. [PubMed]
- Agarwal R. Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant 2012;27:3908-14. [PubMed]
- Fabbian F, Cantelli S, Molino C, et al. Pulmonary hypertension in dialysis patients: a cross-sectional italian study. Int J Nephrol 2010;2011:283475.
- Havlucu Y, Kursat S, Ekmekci C, et al. Pulmonary hypertension in patients with chronic renal failure. Respiration 2007;74:503-10. [PubMed]
- Yigla M, Nakhoul F, Sabag A, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest 2003;123:1577-82. [PubMed]
- Amin M, Fawzy A, Hamid MA, et al. Pulmonary hypertension in patients with chronic renal failure: role of parathyroid hormone and pulmonary artery calcifications. Chest 2003;124:2093-7. [PubMed]
- Nakhoul F, Yigla M, Gilman R, et al. The pathogenesis of pulmonary hypertension in haemodialysis patients via arterio-venous access. Nephrol Dial Transplant 2005;20:1686-92. [PubMed]
- Unal A, Sipahioglu M, Oguz F, et al. Pulmonary hypertension in peritoneal dialysis patients: prevalence and risk factors. Perit Dial Int 2009;29:191-8. [PubMed]
- Kumbar L, Fein PA, Rafiq MA, et al. Pulmonary hypertension in peritoneal dialysis patients. Adv Perit Dial 2007;23:127-31. [PubMed]
- Casas-Aparicio G, Castillo-Martínez L, Orea-Tejeda A, et al. The Effect of Successful Kidney Transplantation on Ventricular Dysfunction and Pulmonary Hypertension. Transplant Proc 2010;42:3524-8. [PubMed]
- Abedini M, Sadeghi M, Naini AE, et al. Pulmonary hypertension among patients on dialysis and kidney transplant recipients. Ren Fail 2013;35:560-5. [PubMed]
- Abassi Z, Nakhoul F, Khankin E, et al. Pulmonary hypertension in chronic dialysis patients with arteriovenous fistula: pathogenesis and therapeutic prospective. Curr Opin Nephrol Hypertens 2006;15:353-60. [PubMed]
- Bozbas SS, Akcay S, Altin C, et al. Pulmonary hypertension in patients with end-stage renal disease undergoing renal transplantation. Transplant Proc 2009;41:2753-6. [PubMed]
- Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43-54. [PubMed]
- Cury JL, Brunetto AF, Aydos RD. Negative effects of chronic kidney failure on lung function and functional capacity. Rev Bras Fisioter 2010;14:91-8. [PubMed]
- Karacan O, Tutal E, Colak T, et al. Pulmonary function in renal transplant recipients and end-stage renal disease patients undergoing maintenance dialysis. Transplant Proc 2006;38:396-400. [PubMed]
- Violan MA, Pomes T, Maldonado S, et al. Exercise capacity in hemodialysis and renal transplant patients. Transplant Proc 2002;34:417-8. [PubMed]
- McIntyre CW, Selby NM, Sigrist M, et al. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant 2006;21:2210-6. [PubMed]
- Dabestani A, Mahan G, Gardin JM, et al. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol 1987;59:662-8. [PubMed]
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009;119:2250-94. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511-22. [PubMed]
- Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720-35. [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [PubMed]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377-81. [PubMed]
- Dagli CE, Sayarlioglu H, Dogan E, et al. Prevalence of and factors affecting pulmonary hypertension in hemodialysis patients. Respiration 2009;78:411-5. [PubMed]
- Tarrass F, Benjelloun M, Medkouri G, et al. Doppler echocardiograph evaluation of pulmonary hypertension in patients undergoing hemodialysis. Hemodial Int 2006;10:356-9. [PubMed]
- Harp RJ, Stavropoulos SW, Wasserstein AG, et al. Pulmonary hypertension among end-stage renal failure patients following hemodialysis access thrombectomy. Cardiovasc Intervent Radiol 2005;28:17-22. [PubMed]
- Akmal M, Barndt RR, Ansari AN, et al. Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int 1995;47:158-63. [PubMed]
- Lee HY, Stretton TB, Barnes AM. The lungs in renal failure. Thorax 1975;30:46-53. [PubMed]
- Bush A, Gabriel R. Pulmonary function in chronic renal failure: effects of dialysis and transplantation. Thorax 1991;46:424-8. [PubMed]
- Herrero JA, Alvarez-Sala JL, Coronel F, et al. Pulmonary diffusing capacity in chronic dialysis patients. Respir Med 2002;96:487-92. [PubMed]
- Eddahibi S, Humbert M, Sediame S, et al. Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension. Effect of prostacyclin therapy. Am J Respir Crit Care Med 2000;162:1493-9. [PubMed]
- Mata-Greenwood E, Meyrick B, Soifer SJ, et al. Expression of VEGF and its receptors Flt-1 and Flk-1/KDR is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2003;285:L222-31. [PubMed]
- Fukumura D, Xu L, Chen Y, et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res 2001;61:6020-4. [PubMed]
- Elias AP, Dias S. Microenvironment changes (in pH) affect VEGF alternative splicing. Cancer Microenviron 2008;1:131-9. [PubMed]
- Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 2006;99:675-91. [PubMed]
- Yuan J, Guo Q, Qureshi AR, et al. Circulating vascular endothelial growth factor (VEGF) and its soluble receptor 1 (sVEGFR-1) are associated with inflammation and mortality in incident dialysis patients. Nephrol Dial Transplant 2013;28:2356-63. [PubMed]
- Adams GR, Vaziri ND. Skeletal muscle dysfunction in chronic renal failure: effects of exercise. American journal of physiology. Renal physiology 2006;290:F753-61. [PubMed]
- Oh-Park M, Fast A, Gopal S, et al. Exercise for the dialyzed: aerobic and strength training during hemodialysis. Am J Phys Med Rehabil 2002;81:814-21. [PubMed]
- Caglar K, Peng Y, Pupim LB, et al. Inflammatory signals associated with hemodialysis. Kidney Int 2002;62:1408-16. [PubMed]
- Ikizler TA, Pupim LB, Brouillette JR, et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab 2002;282:E107-16. [PubMed]
- Simmons EM, Langone A, Sezer MT, et al. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation 2005;79:914-9. [PubMed]
- Guleria S, Agarwal RK, Guleria R, et al. The effect of renal transplantation on pulmonary function and respiratory muscle strength in patients with end-stage renal disease. Transplant Proc 2005;37:664-5. [PubMed]


