Glutathione and nitrite levels in induced sputum at COPD patients and healthy smokers
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality throughout the world. The pathophysiology of airway obstruction in COPD is multifactorial, and involves neutrophilic airway inflammation, protease-antiprotease imbalance, oxidative stress, T cell predominant interstitial inflammation, and recurrent infection (1-4).
The major risk factor for COPD is cigarette smoking, which is one of the most potent oxidants. Other factors that may exacerbate COPD, such as air pollutants, infections, and occupational dusts also have the potential to produce oxidative stress (5).
Oxidants present in cigarette smoke can stimulate alveolar macrophages to produce reactive oxygen species and to release a number of mediators, some of which attract neutrophils and other inflammatory cells into the lungs. Neutrophils and macrophages are known to migrate in increased numbers into the lungs of cigarette smokers, compared to nonsmokers and can generate reactive oxygen species via the NADPH oxidase system (6).
Activated inflammatory cells are also an important source of free radicals in the lungs (7). To scavenge these radical species, airway surfaces are covered with a thin liquid film (epithelial lining fluid) containing a number of antioxidants. One of these antioxidants is glutathione, a tripeptide containing an SH group (8,9). Determining airway glutathione content is of use when investigating oxidative stress in the lung (10).
Nitric oxide (NO) has been used as a marker of airway inflammation and indirectly as a measure of oxidative stress. After production, NO can be exhaled, metabolized to nitrite and nitrate (NO2, NO3), or interact with superoxide to form peroxynitrite. The reaction of NO with O2.– limits the usefulness of this marker in COPD, except perhaps to differentiate from asthma (5,11).
Airway inflammation in COPD can be demonstrated by examination of induced sputum. Sputum induction is a safe and successful method in subjects with COPD patients (12). It is particularly useful as a noninvasive technique when expiratory airflow limitation precludes airway sampling via bronchoscopy (10).
The aim of this study was to assess oxidative stress in stable COPD patients and healthy smokers on induced sputum glutathione, NO2, and serum NO2 contents.
Materials and methods
Subjects
A total of 40 subjects were studied (Table 1).

Full table
COPD patients
Twenty-one COPD patients who satisfied the GOLD criteria (13) were randomly selected from the outpatient clinic of our hospital. They had a history of smoking (>20 pack years) and irreversible airflow limitation [reversibility <10% predicted forced expiratory volume in one second (FEV1) after 200 µg inhaled salbutamol]. They received no medication during the spirometric study and sputum induction. Ten patients (group 1) were clinically stable and none had a history of respiratory infection for at least 4 weeks before the study. Eleven patients (group 2) were studied within 3 hours of admission to the hospital with acute exacerbation of their condition. Exacerbation of COPD was defined by GOLD (13) (increased breathlessness, cough, and sputum production). None of the patients was taking antioxidant medication, which could affect bronchial glutathione levels.
Healthy smokers and non-smokers subjects
A total of 19, age-matched, healthy volunteers [nine smokers (group 3) and ten lifelong non-smokers (group 4)] were randomly selected from the hospital staff. None of the subjects had a history of respiratory or allergic disease, all of their baseline spirometric parameters were normal as predicted for age, sex, and height, none had a history of upper respiratory tract infection in the previous 6 weeks, and none was taking regular medication.
This study was approved by the local ethics committee, and all subjects gave written informed consent.
Pulmonary function tests
Pulmonary function parameters (FEV1, FVC) were measured with SuperSpiro spirometer (Micromedical Limited, UK).
Sputum induction
Sputum induction was performed according to a method previously described (10). Before and after sputum induction, spirometric analysis was performed. In addition, the safety of this procedure was monitored by measuring peak expiratory flow rates (PEFR). If PEFR decreased by 25% the procedure would have been stopped.
Sputum induction was performed by having the subject inhale hypertonic saline (3% NaCl) for maximum 20 minutes from an ultrasonic nebuliser (Porta-Neb compressor, Medic-Aid Sidestream nebuliser chamber, mass median diameter 3.18 µm (Medic-Aid Limited, UK)). Before each sputum expectoration, all subjects rinsed their mouths with distilled water. The first portion of sputum was discarded, and the inhalation procedure was continued for maximum 20 minutes longer. At least five sputum samples were obtained from each subject. Process was terminated before 20 minutes ıf collected enough samples. Expectorated sputum was collected in sterile plastic tubes and placed on ice to slow down metabolic processes, which might result in loss of glutathione.
Sample processing
Sputum samples were processed within 30 minutes of collection. Volumes were measured, and visible salivary contamination was removed from sputum. Samples were diluted with three volumes of chilled phosphate buffered saline (PBS: all reagents were purchased from Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and dispersed by gently agitating the tube and aspirating the sample with a wide bore pipette. Supernatants were obtained by centrifugation (300 g, 15 minutes, 4 °C) and transferred to another vial by filtering through multiple layers of cotton gauze. Additional centrifugation (800 g, 5 minutes, 4 °C) ensured the removal of the remaining cell debris and mucus. Aliquots of the supernatants were placed on ice and assayed immediately for glutathione.
The pellet was diluted with three volumes (per gram of pellet) of freshly prepared 6.5 mM dithiothretiol (DTT) in PBS, vortexed, and incubated at 37 °C for 15 minutes with occasional mixing. The cell suspension was dispersed, filtered through two layers of cotton gauze, and pelleted by centrifugation (300 g, 10 minutes, 25 °C). An aliquot of the cells was cytospin at 1,500 g for 3 minutes at 25 °C (Cytopsin 3, Shandon, Frankfurt/Main, Germany). Cytospins were stained with May-Gruenwald-Giemsa dye and blindly analyzed by a pathologist blind to the clinical characteristics. At the first reading, the percentage of squamous cells was determined, and at the second reading differential cell counts of non-squamous cells (ciliated cells, macrophages, neutrophils, lymphocytes, eosinophils) were evaluated. The squamous cells, bronchial epithelial cells, macrophages, neutrophils, eosinophils, lymphocytes, ciliated cells were counted in ten area on slide by objective (Olympus Bx50), and then the mean counts of each area were taken. The cases without bronchial epithelial cell and/or alveolar macrophages were excluded in evaluation. Only sputum samples with non-squamous cell viability of more than 60% and 60% or fewer squamous cells (<50%) were analyzed. Any samples of sputum contaminated with blood were excluded from analyses.
Glutathione measurement
The total glutathione level of induced sputum samples was measured using an enzymatic recycling assay (14,15). The standard and sample solutions were added to an equal volume of DTNB and 50 µL of this mixture (final concentrations of DTNB 0.25 mM) were pipetted into a 1 mL cuvette, followed by glutathione reductase and NADPH (final concentrations 1 U/mL and 0.22 μM respectively). The reaction mixture was equilibrated, and the kinetic reaction was followed for two minutes at 412 nm (Techcomp Ltd., UV-VIS 8500 spectrophotometer, Hong Kong).
NO2– measurement
Nitrite was measured in sputum and plasma samples, as previously described, using the Griess reaction (16).
Statistical analysis
Data were analyzed using the statistical package for the social sciences (SPSS) software statistical program. Results were given as group means ± standard deviations (SD). Statistical analysis was performed using nonparametric Kruskal-Wallis 1-way ANOVA test for multiple-group comparisons; Mann-Whitney U test was performed to test any observed differences for significance. The significance of correlations was evaluated by determining Spearman rank correlation coefficients. A Pvalue of <0.05 was considered statistically significant.
Results
Sputum induction
All subjects tolerated the sputum induction well. Only a very small decrease in FEV1 was observed in stable COPD patients upon induction (P>0.05, Table 1), and there were no further complications.
Cell counts in sputum
The differential cell percentages in all groups appear in Table 2. The percentage of neutrophils was significantly higher in COPD patients than healthy smokers and non-smokers (P<0.001). The highest percentage of neutrophils was in the acute exacerbations. There were no significant differences in the percentage of lymphocytes, eosinophils, and ciliated cells in the four groups.

Full table
Glutathione and NO2 contents
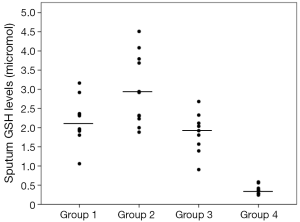
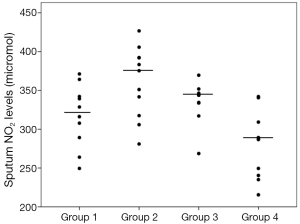
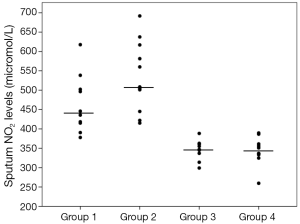
Sputum glutathione, NO2, and serum NO2 levels are shown in Table 3 and Figures 1-3. The highest sputum glutathione, NO2, and serum NO2 levels were in the acute exacerbations of COPD patients. Sputum glutathione levels were significantly lower in non-smoker subjects than in stable and acute exacerbations of COPD patients and the healthy smoker group (P<0.001).

Full table
The NO2 contents of sputum samples in both COPD groups (stable and acute exacerbations) were statistically significantly higher than in the smoker and non-smoker groups (P<0.001).
Although the sputum glutathione and NO2 levels in acute exacerbations of COPD patients were higher than stable COPD patients, statistical analyses were not significant.
Serum NO2 levels in the acute exacerbations group were significantly higher than the stable COPD and non-smoker groups (P<0.05 and P<0.001 respectively). When compared to the stable COPD and healthy smoker groups, no significant differences were determined.
Given only the patients with COPD, it has been found that there is no correlation between the levels of sputum glutathione, NO2, and serum NO2 and sputum neutrophil percentages (r=0.020, r=0.341, and r=0.346, respectively; P>0.05 for all parameters). Similarly, it has been found that there is no relationship between these parameters to FEV1 values (r=–0.218, r=–0.376, and r=–0.243, respectively; P> 0.05 for all parameters).
Discussion
This study was conducted with COPD patients during a stable and acute exacerbation period. Smoker and non-smoker healthy volunteers revealed that oxidative stress was increased in COPD patients. We observed that oxidative stress was also increased in the healthy smoker subjects, although not as significant as in the COPD patients.
COPD is an inflammatory disease, which is characterized by progressive airflow restriction and chronic inflammation affecting the alveoli. Neutrophils are the most significant cells responsible for inflammation. Oxidative stress is the key factor in pathogenesis of the disease. Activated inflammatory cells in the airways, air pollution and smoking are oxidant sources. Previous studies indicated that neutrophil ratio increased in induced sputum in COPD patients and was directly proportional with oxidative stress. Therefore, in the future, neutrophils may be a potential therapeutic target in COPD patients (17,18). Supporting this finding, Serviddio et al. (19) found that eosinophils were greater and oxidative stress was lower in COPD patients with reversibility. In our study, we also found that neutrophil ratio increased both in stable COPD patients and in COPD patients during the exacerbation period.
NO is one of the most important oxidative substances in airways. NO levels were detected to be high in the exhalation air of asthma patients. However, a difference could not be found between healthy subjects and particularly stable COPD patients (17,20). Whereas, NO products were showed an increase in induced sputum of COPD patients. Another study revealed that peroxynitrit inhibitor activity decreased in sputum (21). In another study, NO products increased as exhaled NO level decreased, while disease severity increased in stable patients (22). Therefore, measurement of NO products in sputum seems to be a better method than NO measurement in exhalation air to indicate oxidative stress in COPD patients. In our study, we saw that both sputum and serum NO2 levels were high in COPD patients. Interestingly, we detected that serum NO2 levels were as high in COPD patients as they were in healthy smoking subjects. This result suggests that smoking leads to systemic oxidative stress, although clinically evident airway restriction has not yet developed. In the study of Rahman et al. (23), superoxide anion production was found to increase in neutrophils obtained from the peripheral blood of COPD patients during the acute exacerbation period and decreased to normal levels in the stable period. Similarly, in our study, serum NO2 levels were very high in the exacerbation period and reduced significantly in the stable period. The difference was statistically significant.
Ziora et al. (22) found that there was no association between sputum NO2 products and FEV1 values. This study was conducted only in patients in the stable period. Another study showed a negative correlation between exhaled NO levels and FEV1 levels in stable COPD patients (24). Brindicci et al. (25) detected a negative correlation between alveolar NO levels and FEV1 values and reported that alveolar NO levels could reflect inflammation and remodeling in the periphery of the lungs. In our study, although sputum and serum NO2 levels statistically are higher than healthy individuals, statistically no significant relationship between FEV1 and these parameters was not detected in COPD patients. This result has shown that sputum and serum NO2 levels alone cannot be used to suggest the degree of airway obstruction. However, it should be noted that our study population is small and our patients had advanced diseases.
Glutathione is one of the most important substances as an antioxidant against oxidative load in the lungs. In fact, glutathione is the main molecule of the intracellular antioxidant system. Extracellular glutathione level is very low. However, it is different in the lungs. A very thin respiratory tract lining fluid (RTLF) was detected in studies. RTLF mainly includes an ample amount of protein and lipid (like surfactant). This fluid is also rich in antioxidants, mainly glutathione. Measurement of glutathione levels in the airways is quite beneficial for investigating oxidative stress in the lungs (26,27).
Antioxidant production increases as a defense mechanism as oxidative stress increases. An increase in BAL glutathione levels were detected in chronic smokers (28). An increase was found in sputum glutathione levels in studies conducted with COPD patients (29). In the study of Drost et al. (30), BAL glutathione levels were shown to increase in stable COPD patients and healthy smokers, and it was shown to decrease during the severe and very severe exacerbation periods. Sputum glutathione levels were high in COPD patients in our study, too. However, different from other studies, we detected the highest glutathione levels in cases in the exacerbation period. This finding may be an indication of the increased antioxidant response from increased oxidative stress.
The fact that glutathione loses its stability in a very short amount time is one of the challenges of glutathione level measurement in induced sputum. In a study, sputum glutathione levels were found to be effected from time and were reduced statistically significantly at the 4th hour following sample collection. Therefore, it is recommended to examine sputum glutathione levels as quickly as possible and to freeze the samples until examined (31). Therefore, we initiated examination procedures within 30 minutes of sample collection.
Sputum induction is a method frequently used to show inflammation in the airways. It is preferred in many studies concerning pulmonary diseases, as it is non-invasive. It is reported to be a reliable method (10,12). Our study also revealed that FEV1 values did not show a statistically significant difference after sputum induction.
In summary, we detected that oxidative stress increased both in the stable and exacerbation periods of COPD patients, and oxidative stress developed in healthy smokers, although clinically evident airway restriction hadn’t yet developed. In addition, we may state that sputum induction method is a successful, easy, and reliable method to examine oxidative stress in this patient group.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis 2011;6:413-21. [PubMed]
- Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med 1997;156:341-57. [PubMed]
- Saetta M, Baraldo S, Corbino L, et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:711-7. [PubMed]
- Bresser P, Out TA, van Alphen L, et al. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic haemophilus influenzae airway infection. Comparison with noninfected patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:947-52. [PubMed]
- MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 2001;429:195-207. [PubMed]
- Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med 1996;21:669-81. [PubMed]
- Mak JC. Pathogenesis of COPD. Part II. Oxidative-antioxidative imbalance. Int J Tuberc Lung Dis 2008;12:368-74. [PubMed]
- Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol 1999;277:L1067-88. [PubMed]
- Cantin AM, Bégin R. Glutathione and inflammatory disorders of the lung. Lung 1991;169:123-38. [PubMed]
- Dauletbaev N, Rickmann J, Viel K, et al. Glutathione in induced sputum of healthy individuals and patients with asthma. Thorax 2001;56:13-8. [PubMed]
- Sugiura H, Ichinose M. Nitrative stress in inflammatory lung diseases. Nitric Oxide 2011;25:138-44. [PubMed]
- Brightling CE, Monterio W, Green RH, et al. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatability. Respir Med 2001;95:999-1002. [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Updated 2010:1-92.
- Akerboom TP, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol 1981;77:373-82. [PubMed]
- Buhl R, Vogelmeier C, Critenden M, et al. Augmentation of glutathione in the fluid lining the epithelium of the lower respiratory tract by directly administering glutathione aerosol. Proc Natl Acad Sci U S A 1990;87:4063-7. [PubMed]
- Verdon CP, Burton BA, Prior RL. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal Biochem 1995;224:502-8. [PubMed]
- Zhou M, Liu Y, Duan Y. Breath biomarkers in diagnosis of pulmonary diseases. Clin Chim Acta 2012;413:1770-80. [PubMed]
- Rytilä P, Plataki M, Bucchieri F, et al. Airway neutrophilia in COPD is not associated with increased neutrophil survival. Eur Respir J 2006;28:1163-9. [PubMed]
- Serviddio G, Carpagnano GE, Rollo T, et al. Evidence of lower oxidative stress in the air spaces of patients with reversible COPD. Int J Immunopathol Pharmacol 2006;19:617-28. [PubMed]
- Al-Ali MK, Howarth PH. Exhaled nitric oxide levels in exacerbations of asthma, chronic obstructive pulmonary disease and pneumonia. Saudi Med J 2001;22:249-53. [PubMed]
- Kanazawa H, Shiraishi S, Hirata K, et al. Imbalance between levels of nitrogen oxides and peroxynitrite inhibitory activity in chronic obstructive pulmonary disease. Thorax 2003;58:106-9. [PubMed]
- Ziora D, Dworniczak S, Kaczmarczyk G, et al. Correlation of exhaled nitric oxide with nitrogen oxides and selected cytokines in induced sputum of chronic obstructive pulmonary disease patients. J Physiol Pharmacol 2007;58 Suppl 5:791-9. [PubMed]
- Rahman I, Skwarska E, MacNee W. Attenuation of oxidant/antioxidant imbalance during treatment of exacerbations of chronic obstructive pulmonary disease. Thorax 1997;52:565-8. [PubMed]
- Ansarin K, Chatkin JM, Ferreira IM, et al. Exhaled nitric oxide in chronic obstructive pulmonary disease: relationship to pulmonary function. Eur Respir J 2001;17:934-8. [PubMed]
- Brindicci C, Ito K, Resta O, et al. Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J 2005;26:52-9. [PubMed]
- Kelly FJ. Gluthathione: in defence of the lung. Food Chem Toxicol 1999;37:963-6. [PubMed]
- Cantin AM, North SL, Hubbard RC, et al. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987;63:152-7. [PubMed]
- Morrison D, Rahman I, Lannan S, et al. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med 1999;159:473-9. [PubMed]
- Beeh KM, Beier J, Koppenhoefer N, et al. Increased glutathione disulfide and nitrosothiols in sputum supernatant of patients with stable COPD. Chest 2004;126:1116-22. [PubMed]
- Drost EM, Skwarski KM, Sauleda J, et al. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005;60:293-300. [PubMed]
- Beier J, Beeh KM, Kornmann O, et al. Stability of glutathione in induced sputum: impact of freezing. Respiration 2003;70:523-7. [PubMed]