PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST)
Introduction
Morphological analysis based on CT is primary method in evaluation of treatment response for non-small cell lung cancer (NSCLC) and other solid tumors. Response evaluation criteria in solid tumors (RECIST) is the “gold” criteria in CT evaluation which was established in 2000 and revised in 2009 (RECIST 1.1) (1).With the popularity of PET/CT many researchers have studied the changes of standardized uptake value (SUV) before and after treatment, but there are no uniform criteria for evaluation of treatment response. In 2009 Wahl et al. proposed the PET response criteria in solid tumors (PERCIST) as a new method in which the treatment response was evaluated by metabolic changes (2). The present study was designed to evaluate the therapeutic response of forty-four NSCLC patients according to PERCIST protocol and to compare with the RECIST 1.1 criteria. Further to access PERCIST criteria and discuss the advantage of it relative to RECIST.
Methods
Patients information
With the approval of Ethics Review Board in our hospital the records of NSCLC patients on PACS (Picture Archiving and Communication Systems) were retrospectively reviewed who underwent 18F-FDG PET/CT examination twice or more without operation between Jan 2010 and Jun 2013. The patients were histologically confirmed NSCLC and received chemotherapy which consisted of cisplatin and another drug (such as pemetrexed and so on). After the chemotherapy targeted drugs might be used. The first time of PET/CT examination was before the start of treatment and the second was in 15-30 days after 2 or 4-6 cycles’ chemotherapy. The images met the criterion of RECIST and PERCIST and at least one target lesion could be confirmed. The SULpeak (SUV normalized to body weight and lean body mass) of target lesions at baseline (pretreatment) must not less than (1.5× mean liver SUL + 2SDs of mean SUL). According to the above requirements 44 patients were collected (33 men and 11 women; median age, 67 years; range, 41-83 years; mean weight, 66.3±12.9 kg, range, 43-98 kg).
PET/CT protocol
PET/CT examination was performed with an integrated scanner (Siemens biograph 16). 18F-fluorodeoxyglucose (18F-FDG) was produced by CTI RDS III cyclotron (GE) and the radiochemical purity was more than 95%. Each patient had to fast for 6 hours at least and the blood glucose level must be less than 200 mg/dL before intravenous injection of 18F-FDG at the dose of 3.7-5.5 MBq/kg body weight and been suggested to drink about 1,000 mL water after injection. PET/CT scan was begun about 60 min after injection and the range was from the skull base to the middle of the femur. CT acquisition parameters were as follows: 120 kV and 200 reference mAs; dynamic dose control mode (Caredose 4D); 1.5-mm detector collimation and 5.0-mm slice thickness. PET parameters: 3D emission scan, 1.5-2 min per bed position; 6-7 beds, ordered-subset expectation maximization (OSEM) reconstruction. CT scan data was used for attenuation correction of PET image. Breath-holding CT images including lung lesions were obtained after PET/CT program and thin-section images were reconstructed.
Target lesions and measurement
The target lesions of patients on PET/CT images were determined by two experienced radiologists. Only one target lesion was chosen in the present study because there is one target lesion in PERCIST protocol, and this might be more comparable for PERCIST and RECIST. The target lesion size (length × width) was measured on breath-holding CT mediastinal window images and recorded as CT baseline data. The peak SUL of hottest single tumor lesion with maximal 1.2-cm diameter volume ROI (SULpeak) was required to measure in PERCIST. The software on Siemens PET/CT wizard workstation had limitations in obtaining the peak SUV directly. In this study we used layer by layer accumulated region of interest (ROI) measurement method by reference to the related literatures (3,4). At the center layer of lesion (including the maximal SUV) the ROI with 1.2-cm diameter was made and the mean SUV of three continuous layers (layer thickness was about 4 mm) adjoin to the centre layer were measured. The average of three mean SUVs was approximately taken as the peak SUV (SUVpeak) of volumetric ROI. Then the SUVpeak was normalized for the lean body mass and generated SULpeak According to the formula as follows (5): SUL = A/(ID/LBM), LBM (male) =1.10× BW − 120 (BW/H)2, LBM (female) =1.07× BW − 148 (BW/H)2. Where A is the decay-corrected tissue activity concentration (measured in megabecquerels per milliliter), ID is the net injected dose (in megabecquerels), BW is the patient’s body weight (in grams), and LBM is the patient’s lean body mass. The longest diameters and SULpeak of target lesions on the PET/CT images before and after treatment were measured and recorded as D1, D2 and SUL1, SUL2.
Response evaluation methods
Objective therapeutic responses according to RECIST 1.1 are as follows (1): complete remission (CR) is disappearance of target lesion for at least 4 wk; partial remission (PR) is a decline of at least 30% in tumor diameter; stable disease (SD) is neither PR nor progressive disease (PD); and PD is at least a 20% increase in tumor diameter and 5-mm absolute increase was required. Objective therapeutic responses according to PERCIST 1.0 are as follows (2): complete metabolic response (CMR) is complete resolution of 18F-FDG uptake within the measurable target lesion so that it is less than mean liver activity and indistinguishable from surrounding background blood-pool levels with no new 18F-FDG-avid lesions. Partial metabolic response (PMR) is reduction of a minimum of 30% in the target tumor 18F-FDG SULpeak. Stable metabolic disease (SMD) is disease other than CMR, PMR, or progressive metabolic disease (PMD); and PMD is a 30% increase in 18F-FDG SULpeak or advent of new 18F-FDG-avid lesions that are typical of cancer.
Statistical and survival analysis
The percentage changes of longest diameters and SULpeak of target lesions in 44 patients before and after treatment were calculated according to the formula as follows: ΔD%= (D1 – D2)/D1 ×100%, ΔSUL% = (SUL1 – SUL2)/SUL1 ×100%. A paired Student’s t-test method was used to assess the statistical significance of these two changes and the results could evaluate the sensitivity of CT and PET on the response. Then the response was classed into four levels according to RECIST and PERCIST: CR (CMR) =1, PR (PMR) =2, SD (SMD) =3, PD (PMD) =4. Pearson chi-square test was used to compare the proportion of four levels in RECIST and PERCIST. Because the new lesions noted on PET/CT were used for progress in RECIST 1.1, in order to compare PET/CT and CT in the evaluation of treatment response, the new lesions which could not be found or confirmed on routine CT were eliminated in RECIST and compared with PERCIST once more with chi-square test. A P value of less than 0.05 was considered to be significant.
The relationship between progression-free survival (PFS) and clinicopathologic results (such as TNM stage, percentage changes, RECIST and PERCIST results etc.) were evaluated using univariate Cox proportional hazards regression analysis. Significant parameters identified by univariate analysis were included in a multivariate Cox proportional hazards regression analysis [stepwise selection (Wald) method; P≤0.05 was used for entry into the model, and P>0.1 was selected for removal]. The statistical software was SPSS17.0.
Results
The relation between the changes of diameter and SUL
There were 30 adenocarcinoma and 14 squamous cell carcinoma cases in 44 patients. TNM staging were 10 cases in stage II, 7 cases in stage III and 27 cases in stage IV. Twenty-five patients were reviewed at the end of 2 cycles of chemotherapy and 19 patients at the end of 4-6 cycles of chemotherapy. The longest diameters, SUL and percentage changes of target lesions before and after treatment were shown in Table 1 and Figure 1. The difference of percentage changes between diameter and SUL was not significant using paired t-test (t=–1.69, P=0.098). However if the 40 cases without increasing SUL after treatment were analyzed there was significant difference in the percentage changes between diameter and SUL (t=–3.31, P=0.002).
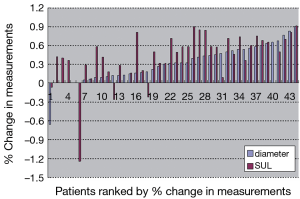
The response evaluation of RECIST and PERCIST
The response classification for 44 patients according to RECIST and PERCIST criteria was as follows: CR/CMR, 2/7; PR/PMR, 21/22; SD/SMD, 15/8; PD/PMD, 6/7; and 15 patients were not consistent. The difference between RECIST and PERCIST was not significant by chi-square test (Pearson χ2=5.008, P=0.171). If the new lesions which could not be found or identified on CT images were revaluated in RECIST, the evaluation results were CR/CMR, 2/7; PR/PMR, 22/22; SD/SMD, 19/8; PD/PMD, 1/7. The grading of 20 patients were not consistent and the difference between RECIST and PERCIST was significant by chi-square test (Pearson χ2=11.759, P=0.007). The details of evaluation results were summarized in Tables 2 and 3.
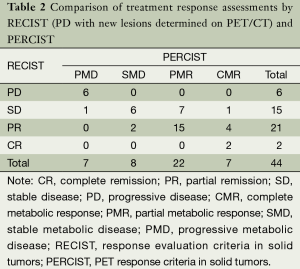
Full table
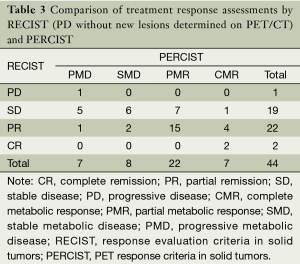
Full table
Survival analysis and prognosis
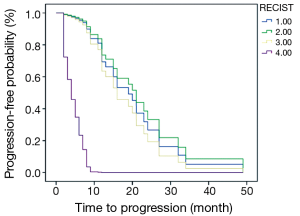
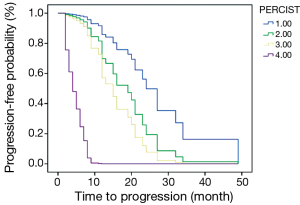
The PFS of 44 patients was 2-49 months and the average was 14.8 months. Associations between PFS and clinicopathologic results, changes of imaging parameters and chemotherapeutic responses [such as TNM stage, reduction rate of tumor diameter (%), chemotherapy cycles (2 or 4-6), reduction rate of SULpeak (%), RECIST (CR/PR/SD/PD) and PERCIST (CMR/PMR/SMD/PMD)] were assessed using univariate and multivariate Cox proportional hazards regression analysis (Table 4). Reduction rate of SULpeak (%), RECIST and PERCIST were significant factors in univariate Cox analysis. But Multivariate Cox proportional hazards regression analysis demonstrated that only PERCIST was a significant factor for predicting DFS [hazard ratio (HR), 3.20; 95% CI: 1.85-5.54; P<0.001]. The survival curve of RECIST and PERCIST produced by SPSS was shown in Figures 2 and 3.

Full table
Discussion
RECIST criteria is widely applied to evaluate the treatment response for solid tumors, but is known to have limitations because it depends on the morphologic changes (2,6). Now with increasing use of the targeted therapy, such as antiangiogenic therapy in clinic, a new evaluation method is necessary to effectively monitor the response of this therapy (6). 18F-FDG PET or PET/CT is considered to overcome such limitations and more suitable for assessment of therapeutic effect because it can better reflect the intrinsic nature of malignant tumor (7). The present study demonstrated that the percentage changes of SUL after treatment for NSCLC monitored by PET were higher than the percentage changes of diameter by CT and the evaluation results by PERCIST were more sensitive and prognostic than the evaluation results by RECIST.
Many studies have confirmed that 18F-FDG PET can monitor the metabolic changes of tumors after treatment when the morphologic changes on CT images can not been detected (8,9). The data of most patients in this study also support this viewpoint in which the reduction percentages of diameter were significantly lower than that of SUL on PET/CT images. But no statistically significant result was obtained in 44 patients’ data with paired t-test or non parametric test methods (Wilcoxon signed-rank test). The selection bias should be responsible for this inconformity because of the negative data of progression patients. If the increasing SUL cases and decreasing SUL cases were respectively analyzed, the percentage reduction of SUL in 40 patients was significantly higher than that of diameter. To our knowledge there is no research that proposed the similar problem although a considerable proportion of assessment result was progression in clinic. The reason may be that the similar comparison of percentage changes was not studied by the other research. However the classification according to RECIST or PERCIST or others is established by the researchers rather than tumors itself and it may be a problem when the reduction of 29% is compared with the reduction of 31%. Further research should pay attention to the details of information in the response assessment.
In the present study the evaluation results were not significantly different between PERCIST and RECIST 1.1 (with new lesions determined on PET/CT), but showed significant difference between PERCIST and RECIST 1.0 (without new lesions determined on PET/CT). This result revealed that PERCIST and RECIST 1.1 had good consistency and PERCIST (or PET) was more sensitive in detection the CR and progression patients. In the study of Van Ruychevelt et al. 59 NSCLC patients were evaluated by RECIST and EORTC criteria, and the results showed that PET was more sensitive than CT in early detecting the patients of PD (10). In the research of Yanagawa et al. Fifty-one patients with locally advanced esophageal cancer who received neoadjuvant chemotherapy were studied. Chemotherapeutic lesion responses were evaluated using 18F-FDG PET and CT according to the RECIST and PERCIST methods. There was significant difference between the PERCIST and RECIST evaluation results and the number of CR cases in PERCIST was much more than which in RECIST (4). All these studies indicated that PERCIST is superior to RECIST in the detection of CR and progression. One possible reason is that the metabolic changes after treatment is more sensitive than morphologic changes in the nature of tumors and PET can just monitor the metabolic changes. The other reason may be that the intrinsic properties of this two criteria because the achievement to CR in RECIST is more difficult. In the ordinary PET/CT work we found that some NSCLC lesions didn’t further shrank or disappear when they reduced to a certain degree but no uptake of 18F-FDG. The residual lesions may be the fiber texture or scar tissue and can be confirmed by surgery.
The relationship between the metabolic changes of tumors and prognosis was discussed in many studies. The prognostic value of parameters about SUV and the evaluation results was not in agreement (8-10). In the study of van Ruychevelt et al. only a significant reduced survival was observed in progressive patients and no differences among the else (10). The other study about Esophageal Cancer concluded that PERCIST 1.0 (CMR vs. non-CMR) was the most significant prognostic factor for predicting DFS and OS in the multivariate Cox proportional hazards regression analysis (4). The results of another study showed that an early metabolic response did not translate into better survival outcome (8). The present study draw a conclusion that only PERCIST evaluation result is a significant prognostic factor and the survival curve suggested that the progressive patients had significantly shorter PFS. The conclusions in the above studies have limitations because of the small number of cases and different classification results. In the further study, the factors affecting the survival and evaluation results [including age, TNM stage, pathological type, subsequent treatment, the basal SUV, Total lesion glycolysis (11) and review time et al.] should be taken into account as far as possible in the multivariate Cox proportional hazards regression analysis, thus the conclusion may be more credible.
Limitations in this study included the retrospective nature of patient data collection, the number of target lesion and the different cycles’ interval of PET/CT review. In PERCIST only one target lesion was required to evaluate but in RECIST 1.1 no more than five target lesions were included. In order to precisely compare PERCIST with RECIST we evaluated the longest diameter of just one target lesion that was assessed in PERCIST. The study of Darkeh MH et al. suggested that measuring fewer than four target lesions might cause discrepancies when more than five target lesions are present in RECIST 1.0 (12). So the evaluation results according to RECIST criteria in the present study might not be accurate. Here a new problem will be proposed that how many target lesions should be chosen when the research aim to compare the PERCIST with RECIST. Another limitation is the time of PET/CT review was not consistent in the present study. Although the cycles’ interval (2 or 4-6 cycles) was not significant factor in the univariate Cox proportional hazards regression analyses and the present study is paired analysis, the difference of sensitivity between PERCIST and RECIST will reduce as the time go on. The further research had better separate the different treatment time of patients and analyze respectively.
Conclusions
In conclusion, RECIST criteria are still a “gold standard” in the response evaluation of the solid tumor. The present study indicates that PERCIST and RECIST 1.1 have good consistency and PERCIST (or PET) is more sensitive in detection the CR and progression patients. Combining the PERCIST and RECIST the clinician will acquire more response information relatively early. However because of the small number of patients the selection bias could not be avoided. More researchers are expected to join the study of PERCIST and make it serve for tumor patients better.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S-50S. [PubMed]
- Dibble EH, Alvarez AC, Truong MT, et al. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med 2012;53:709-15. [PubMed]
- Yanagawa M, Tatsumi M, Miyata H, et al. Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J Nucl Med 2012;53:872-80. [PubMed]
- Abikhzer G, Alabed YZ, Azoulay L, et al. Altered hepatic metabolic activity in patients with hepatic steatosis on FDG PET/CT. AJR Am J Roentgenol 2011;196:176-80. [PubMed]
- de Langen AJ, van den Boogaart V, Lubberink M, et al. Monitoring response to antiangiogenic therapy in non-small cell lung cancer using imaging markers derived from PET and dynamic contrast-enhanced MRI. J Nucl Med 2011;52:48-55. [PubMed]
- Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med 2006;354:496-507. [PubMed]
- Lee DH, Kim SK, Lee HY, et al. Early prediction of response to first-line therapy using integrated 18F-FDG PET/CT for patients with advanced/metastatic non-small cell lung cancer. J Thorac Oncol 2009;4:816-21. [PubMed]
- Skoura E, Datseris IE, Platis I, et al. Role of positron emission tomography in the early prediction of response to chemotherapy in patients with non--small-cell lung cancer. Clin Lung Cancer 2012;13:181-7. [PubMed]
- van Ruychevelt V, Garcia C, Meert AP, et al. Positron emission tomography with 18F-FDG and cancer response to chemotherapy. Rev Mal Respir 2011;28:618-25. [PubMed]
- Chen HH, Chiu NT, Su WC, et al. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology 2012;264:559-66. [PubMed]
- Darkeh MH, Suzuki C, Torkzad MR. The minimum number of target lesions that need to be measured to be representative of the total number of target lesions (according to RECIST). Br J Radiol 2009;82:681-6. [PubMed]



