Management of malignant pleural mesothelioma—The European experience
Introduction
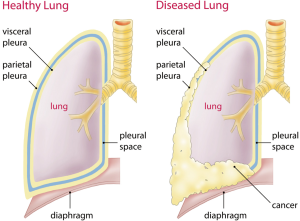
Malignant pleural mesothelioma (MPM) is an aggressive tumor arising from the mesothelial lining cells of the pleura (Figure 1). MPM is a rare cancer, difficult to treat and commonly associated with asbestos exposure [reviewed in (1)]. In Europe, the incidence is about 20 per million with large intercountry variation (2). The incidence is 1.25/100,000 for example in Great Britain and 1.1/100,000 in Germany (3). Since Wagner first recognized the association between asbestos and mesothelioma in 1960 (4), the first round of regulatory measures were prompted only 30 years later in developed countries, beginning in the United Kingdom (UK) and shortly thereafter in the United States (US). Since the median latency between asbestos exposure and disease onset is 44.6 years, based on the Italian Mesothelioma Registry, and increases over time in a linear fashion (5), incidence rates in European nations are still rising, with peak incidences expected around 2020 and beyond (6). Moreover, asbestos is continuously used in many developing countries of the world.
Diagnosis and staging of MPM
A definitive clinical diagnosis of MPM is not generally accepted. Non-specific symptoms such as dyspnea due to pleural effusions with or without chest pain lead in the follow up to the diagnosis of MPM.
Imaging plays an important role in diagnosis, staging, treatment planning (especially in terms of resectability), response assessment, and follow up of MPM patients. Several modalities are available including computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and PET/CT.
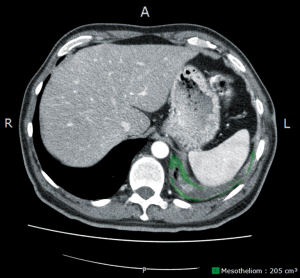
Usually chest radiography is the first imaging to be implemented revealing a unilateral pleural effusion or pleural thickening. The chest CT scan remains the mainstay of the diagnostic tools. Besides the unilateral pleural effusion, circumferential diffuse or nodular pleural thickening, especially of the mediastinal pleura, and contraction of the hemithorax is suggestive of the disease (7-9). The disease tends to progress from the diaphragm to the apex and the fissures are getting involved at a later stage of the disease leading to further infiltration of the underlying lung parenchyma. Clinical staging based on CT scan alone remains a challenge, especially the T-stage. MRI (transdiaphragmatic invasion, multilevel chest wall infiltration) (10) and PET/CT (extrathoracic disease) might help to increase the accuracy of clinical staging, which is usually underestimated, and especially to rule out unexpected distant metastases. Of note, previous talc pleurodesis can influence the interpretation of the PET results. For further treatment planning, a videomediastinoscopy for accurate mediastinal lymph node staging should be considered when a surgical concept within a multimodality program is planned. Endobronchial ultrasound guided techniques (EBUS) for mediastinal staging are currently under evaluation at different centres. According to institutional practice laparoscopy and contralateral VATS may be performed if clinically indicated (11). Response assessment in malignant mesothelioma remains difficult because of the irregular rind-like growth pattern of MPM. Modified RECIST is currently most widely used (12), but criticized for a high inter-observer variability (13). More sophisticated methods such as computerized analysis of CT scans measuring the tumor volume (Figure 2) (14) or PET-CT based algorithms—using the total glycolytic volume or total lesion glycolysis or decrease in SUV-max—which have been demonstrated to have also prognostic value—are under evaluation (15).
Histopathological analysis of pleural tissue is mandatory for final MPM diagnosis but can be difficult because mesothelioma is a heterogeneous cancer and besides this, the pleura is also a common site for metastatic disease. Three principal histotypes can be differentiated according to WHO 2004 classification (16): the epithelioid, the sarcomatoid and a mixture of both—including at least 10% of each growth pattern—the biphasic subtype. The recommendation of the Guidelines of the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) (17) are strongly supporting a thoracoscopic tissue biopsy in order to get multiple and deep tissue biopsy. It has been shown that cytological assessment of pleural effusion may not be sensitive and specific enough (11). Also fine needle biopsies are not primarily recommended because they are associated with low sensitivity (~30%) (11). A conclusive diagnosis can only be made, if the material is representative in terms of biopsy location (normal and abnormal pleura), depth (to assess fat and/or muscle tumor invasion), and quantity (enough material to allow immunohistochemical characterization).
In an attempt to clearly distinguish mesothelioma from adenocarcinoma, numerous monoclonal antibodies with variable sensitivity and specificity have been introduced over the last few decades and have become integral to the diagnosis of malignant mesothelioma. However, an absolutely specific and sensitive marker for mesothelial proliferations has yet to be identified. Currently, a panel of four markers, including two mesothelial and two epithelial antibodies, is used to distinguish epithelioid mesothelioma from non-mesothelial carcinoma. In this context, antibodies with a relatively high sensitivity and/or specificity for mesothelial proliferations include Calretinin, WT-1, and CK5/6, whereas Ber-Ep4, CEA, TTF-1, and MOC-31 are expressed in epithelial neoplasia (Table 1). To differentiate atypical mesothelial hyperplasia from mesothelioma additional markers, such as desmin and EMA, are being used, but their value is hampered by limited sensitivity and specificity. FISH for the detection of p16/CDKN2A deletions has proven to be useful in the distinction between reactive and neoplastic mesothelial proliferations. Since p16 deletions can be detected in up to 70% of cases with pleural mesotheliomas only, FISH for p16, either in isolation or in combination with other markers, does not represent a decisive discriminator between both entities. In the differentiation between malignant mesothelioma and reactive mesothelial proliferations, the demonstration of invasion remains superior to any of the above mentioned markers (11). To separate sarcomatoid mesothelioma from squamous and transitional cell carcinoma, it is recommended to use two broad-spectrum anti-cytokeratin antibodies and two markers with negative predictive value (such as anti-CD34 and anti-B-cell lymphoma 2 marker, anti-desmin, anti-S100) to confirm the diagnosis (11).
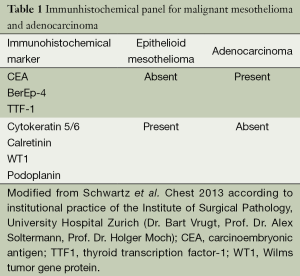
Full table
Pathological staging for MPM was first proposed by Butchart et al. (18), and modified later by Mattson (19) and Sugarbaker et al. (20). The Cancer Staging Manuals of the International Union Against Cancer (UICC) (21) and The American Joint Committee on Cancer (AJCC) (22), first included TNM criteria for MPM in their 4th editions. The International Association for the Study of Lung Cancer (IASLC) and the International Mesothelioma Interest Group (IMIG) developed a TNM-staging system which has been accepted by the UICC and the AJCC (23) and have since remained unchanged through the current (7th) edition. The latest analysis of the IASLC database representing the largest, multicenter and international database on MPM to date and including data from 3,101 patients from 15 centers, mostly from North America and Europe, demonstrates that the proposed TNM staging system effectively distinguishes the T and N categories, but also highlights areas for potential revision in the future (24). Currently IASLC and IMIG are preparing the 8th edition of the TNM classification based on this dataset.
Treatment of MPM
To date, combined treatment modalities are the most commonly used approach for mesothelioma patients in many centres. However, due to the variability of the clinical presentation including histology and stage, the uncertain prognosis and the risk profile of the patient, discussion of every case should be performed within a multidisciplinary team including besides a thoracic surgeon experienced in mesothelioma surgery, a radiation oncologist, an oncologist but also pathologists, respirologists and radiologists.
Surgery for MPM
The role of surgery continues to be important in the diagnosis, staging, and treatment of MPM. Regarding the surgical technique for MPM resection, macroscopic complete resection (MCR) should be the overall aim of the resection. For the time being, there are two main procedures used to obtain MCR—pleurectomy/decortication (P/D) or extrapleural pneumonectomy (EPP). Whereas the surgical technique of EPP has been well standardized with en bloc resection of the parietal and visceral pleura with the ipsilateral lung, pericardium, and diaphragm (25), the technique of P/D is not standardized in all centres as demonstrated during the on-going staging project of the IMIG and the IASLC (26). While some surgeons define P/D as macroscopic tumor removal with pleurectomy of the parietal pleura and decortication of the visceral pleura, others include resection of pericardium and diaphragm involved by the tumor (now recommended by the working group to be nominated as “extended” P/D). The fact, that comparison of both techniques can be based “only” on results available from large institutional reports using different patients’ inclusion criteria, different adjuvant or neoadjuvant protocols and heterogeneous morbidity definition criteria make any evidence based conclusion difficult which procedure—P/D or EPP—is the more appropriate technique to achieve long term survival balanced with best quality of life.
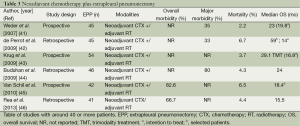
The role of EPP in the treatment of MPM has been recently the subject of debate especially after publication of the Mesothelioma and Radical Surgery (MARS I) trial (27), despite an increasing amount of phase II studies reported favourable results (Tables 2 and 3). The MARS I trial concluded that “EPP within trimodal therapy offers no benefit and possibly harms patients” although the trial included 16 patients in the EPP arm only. The study was not designed to answer the question of benefit or not of EPP but rather of the feasibility of such a trial. A definitive answer to this question would need an accrual of 670 patients to identify a survival benefit (47). Also the criticism of too high morbidity and mortality rate is not supported by recently reported other trials for trimodality therapy (TMT) including EPP showing that morbidity stays high (22-82%) but seems to be manageable in terms of mortality in a range of 2-5% in experienced centres. But taking into account all studies published between 1985-2010 a wide range of 0 to a maximum of 11.8% is reported (48).
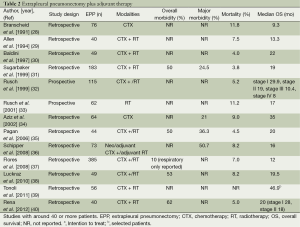
Full table
Full table
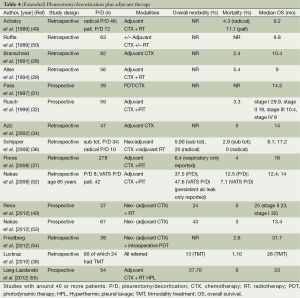
Recently, the Systematic Review Unit of the University in Sydney analysed the surgical treatment of MPM including all relevant data on comparative outcomes of extended P/D (Table 4) and EPP in multimodality settings. In most studies, P/D was usually chosen for earlier stages and EPP for more advanced stages, a decision which is often taken only in the operating theatre due to a lack of reliable clinical staging. Perioperative mortality rates were significantly lower in the P/D group (2.9% vs. 6.8%) and also morbidity rates were lower after the lungs-sparing procedure (27.9% vs. 62% for EPP). However, comparability of morbidity data is complicated by the fact that definition of morbidity is very heterogeneous. Nearly all patients invariably present relevant postoperative air leaks after P/D and a substantial part of these patients suffer from persistent air leak. Most probably this is not reflected in median morbidity rates of 28%. In general, the relatively high morbidity as well as mortality rate indicates the necessity to perform both procedures at a dedicated MPM center requiring not only experienced thoracic surgeons but also routined teams of anaesthesiologists, ICU therapists and nursing teams on the ward.
Full table
Another important aspect to be considered is the Quality of Life (QoL) during and after treatment, but unfortunately there is not much data available. Rena et al. have included QoL data in their 11 years institutional report comparing EPP and P/D and found a superiority of P/D of EPP in QoL after 6 and 12 months (40). However, it has been reported for patients undergoing EPP that an improvement in QoL occurred for all parameters at 3 months postoperatively (56), confirmed by another study showing sustained improvement in QoL after EPP (57). This holds particularly true for patients with shortness of breath because of entrapped lungs or due to an important ventilation/perfusion mismatch.
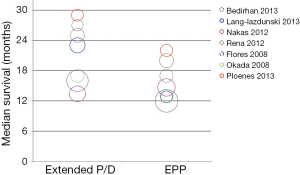
Regarding long-term oncological outcome, initial analysis of the IASLC reported a survival advantage in patients undergoing EPP compared to P/D (58). Cao et al. reported that there was insufficient data to perform a meta-analysis in their systematic review comparing EPP with P/D with respect to overall survival between both procedures. Median overall survival ranged between 13-29 months for extended P/D and 12-22 months for EPP.Figure 3 represents a bubble graph summarizing median survival outcomes in relation to study size published (59). In general, these comparative analyses must be interpreted with caution not only for the heterogeneous definitions for morbidity mentioned beforehand but also for different approaches for survival calculation. Other reasons for differences in outcomes might be subject of a selection bias. In the largest retrospective multicentre study on 663 patients combining the experience of three large centres in the United States, with EPP or P/D by Flores et al. (included in the review by Cao et al.) (37), the authors reported that in general, patients selected for EPP had locally more advanced disease and P/D was applied in earlier stages. The authors conclude that the study emphasizes the similarities in outcome after EPP or P/D for MPM in a multi-centre setting but cannot give a clear recommendation for either one or the other surgical approach. Progression-free survival if reported, is usually longer after EPP in comparison to P/D, and especially the local recurrence rates are higher in P/D groups (37).
Currently a new feasibility study—MARS 2—is open but not yet recruiting in the UK [“MARS 2: A Feasibility Study Comparing (Extended) Pleurectomy Decortication Versus no Pleurectomy Decortication in Patients with Malignant Pleural Mesothelioma” ClinicalTrials.gov Identifier: NCT02040272]. After 2 cycles of induction chemotherapy with cisplatin/pemetrexed, patients will be randomised to receive chemotherapy only (4 cycles of cisplatin/pemetrexed) or lung-sparing surgery plus chemotherapy (4 cycles of cisplatin/pemetrexed). Primary endpoint of the study is the ability to randomise 50 patients within the first 24 months or the ability to recruit 25 patients within a 6 months period.
Taking this information together, there is no clear recommendation which operation is better than the other. The situation where P/D is clearly advised is for patients with compromised cardiac or pulmonary function, or with certain co-morbidities, who would not allow an EPP without excessive risk. Therefore, MPM patients eligible for resection should undergo careful preoperative functional assessment. Pulmonary function testing showing a forced expiratory volume in 1 second (FEV1) of greater than 2 L is generally adequate for pneumonectomy for nearly all patients. Quantitative ventilation/perfusion scanning should be performed and predicted postoperative (PPO) FEV1 (ideally more than 1.2 L) be calculated in all patients if pneumonectomy is not excluded. If both, FEV1 and DLCO are above 80% resection up to pneumonectomy is feasible without any further investigation (60). Cardiac assessment should be performed depending on the patients’ comorbidities, some centres advise routine echography for all patients undergoing EPP in order to rule out pulmonary hypertension.
Parenchyma-sparing debulking P/D or partial pleurectomy should be considered if all gross tumor cannot be removed macroscopically especially in stage IV patients (61) but freeing an entrapped lung would improve respiratory function. This palliative surgical approach is also performed by VATS (62-65) with the intention to improve QoL of these patients. Alternatively, indwelling pleural catheters which can be set-up in an outpatient setting and are easy to handle for the patient, are a very good alternative for a rapid palliation of recurring pleural effusions (66,67). Talc pleurodesis is efficient in prevention of pleural effusion recurrence but requires a full expansion of the lung. Recently the MesoVATS trial has randomized MPM patients to undergo VATS pleurectomy vs. talc pleurodesis via an indwelling intercostal chest drain or via thoracoscopy. Survival rates were about the same but VATS pleurectomy significantly improved control of recurrent build-up of fluid in the lungs in the first 6 months after the procedure, and improved QoL for 12 months (68).
Beside these quite unambiguous situations, P/D and EPP should most probably not be considered as a procedure which serves for all clinical situations. The decision to perform P/D or EPP in stage I, II, and III should be tailored individually by combining patients’ performance status and wish and the extent of the tumor load, and here particularly the degree of fissure involvement limiting the success of P/D substantially and especially may end up with a decorticated lung without relevant residual function. However, there is a controversy among different centers. Some recommend P/D for earlier stages whereas others recommend EPP for these situations (69). Also the further treatment plan influences the procedure choice as adjuvant radiotherapy cannot be applied safely after P/D, because radiation of the intact lungs results most likely in high rates of pneumonitis, even if modern techniques are applied (70). Furthermore, the radiation field is limited to the chest wall and the lung surface only and spares the fissures.
During the last IMIG meeting in Boston 2012, the role of surgery, including both extended pleurectomy/decortication (P/D) and EPP, in the treatment of MPM was critically reviewed. It was agreed under the experts of the field that:
- Surgical MCR and control of micrometastatic disease play a vital role in the multimodality therapy of MPM, as is the case for other solid malignancies;
- Surgical cytoreduction is indicated when MCR is deemed achievable;
- The type of surgery (EPP or P/D) depends on clinical factors and on individual surgical judgment and expertise (71).
The challenge nowadays lies therefore witnin selecting the right patient for the procedure and therapy he potentially benefits the most. Patients with histologically proven mesothelioma and resectable tumor load who would tolerate different treatment modalities including surgery should be considered for a multimodal approach. The clinical staging and functional assessment is mandatory as a basis for this discussion. In many centres only patients with epithelioid type of MPM and without N2 lymph node metastases are considered as candidates for surgery. However, it is proposed that N2 nodes in MPM should be considered more as “local” nodes and therefore are not an exclusion factor per se. Data about the role of mediastinal lymph node involvement of the different case series are conflicting (41,42), but the results of the IASLC/IMIG staging project demonstrates that N2 is not a survival influencing factor (58). Although, sarcomatoid histotype is an exclusion criterion in most clinical trials (11) since it is associated with poor prognosis, there are special forms with slowly growing localized tumors easily resectable which should be at least considered for multimodality treatment. Furthermore, the volume of the tumor is an essential factor for the patient’s prognosis (72,73). The final analysis of extended selection algorithms is pending but is most probably a function of combining several clinical factors, ideally being available before the treatment starts (Opitz, Weder submitted).
Multimodality treatment
According to the Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of MPM, MPM patients should be offered a multimodality treatment approach, ideally within a prospective trial at a specialized center (11).
With regard to chemotherapy, limited chemotherapeutic response rates of only 15% have been reported for single drug use, but recent reviews (74,75) summarize that the antifolate pemetrexed and cisplatin achieve best overall survival and quality of life. Therefore, cisplatin plus an antifolate is currently the most frequently used regimen for first line chemotherapy in a neo- or adjuvant setting. Median survival range for patients who receive cisplatin chemotherapy alone or in combination with pemetrexed is 9.3-13.3 months (76), and for palliative therapy, 7 months (77,78) only. Other chemotherapeutic agents with proven activity against MPM are gemicitabine (79-84) and vinorelbine (85), alone or in combination with cisplatin. Although first line therapy with cisplatin plus pemetrexed remains the standard of care, many novel agents are currently being investigated. Unfortunately, targeted therapies are still far from being applied in patients. However several newer agents aiming at inhibiting specific pathways, that were demonstrated to be present in MPM e.g., PI3Kinase (86,87), are currently under investigation, but biomarker studies to identify the subgroup of patients benefitting from these treatments will be necessary.
Regarding the sequence of multimodality treatment, one particular approach is to perform induction chemotherapy followed by surgery—a concept which has been adapted from stage III NSCLC with the idea to possibly downstage the tumor or eradicate the outer tumor layer for better resectability (41). The concept was studied more in detailed in a Swiss multi-centre trial (41).
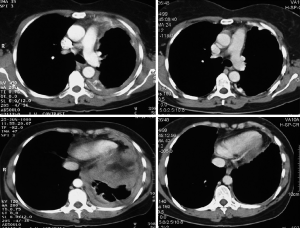
In general, it is expected that chemotherapy would be better tolerated if performed before surgery allowing to apply the full required dose. Another advantage of induction chemotherapy in comparison to adjuvant chemotherapy is the possible downstaging effect on the tumor (Figure 4), which will lead to improved macroscopic complete resection, and better patient selection based on tumor aggressiveness assessed by post-chemotherapy CT scans. Disadvantages are potentially increased surgical mortality and morbidity which is not confirmed in the larger series (see Tables 2 and 3) and the possibility that the delay of surgery may negatively influence resectability. Usually 4-6 weeks after the last cycle of chemotherapy is considered as the optimal time frame for surgery.
The fact that MPM does not respond well to chemotherapy in comparison to other malignancies was confirmed in all neoadjuvant treatment studies, but still, there are a few reports on chemotherapy induced complete pathological response in the literature (88). Unfortunately, the clinical assessment of chemotherapy treatment response is difficult and the only available tool of modified RECIST criteria (12) lacks reliable reproducibility (89). For the time being no validated predictive markers exist for the assessment of mesothelioma chemotherapy response although low thymidilate synthase protein levels were predictive for improved survival in a retrospective analysis of patients who received pemetrexed (90). DNA repair marker such as ERCC1 and RRM1 were demonstrated to have a predictive and independent prognostic role, especially in patient subgroups receiving chemotherapy with cisplatin/gemcitabine (Frischknecht, Opitz et al. submitted).
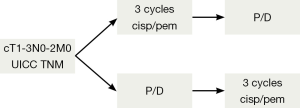
The employment of induction chemotherapy is supported not only by acceptable response rates of 30-40%, but also by convincing resectability rates for EPP after induction chemotherapy reported from prospective trials ranging up to 74% (41,91). The exact value of induction chemotherapy in comparison to adjuvant treatment is difficult to assess. The reported outcome after induction chemotherapy followed by EPP (MST ranging from 14-25.5 months) supports this approach and is comparable to other multimodal concepts such as adjuvant chemotherapy after surgery (MST ranging from 13-24 months [reviewed in (48)]. Tables 2 and 3 summarize the reported literature on EPP with adjuvant or neoadjuvant therapy. Currently the European Organization for Research and Treatment of Cancer (EORTC) will open a new phase II trial soon comparing the neo- vs. the adjuvant setting in MPM patients undergoing P/D (Figure 5).
The role of adjuvant curative intent hemithoracic radiotherapy in MPM remains unclear although a reduction of local recurrence rates has been demonstrated in several series (33,92). The challenges in adjuvant radiotherapy are the big target volumes and the presence of vital structures in the immediate neighborhood (spinal cord, contralateral lung heart and liver). In general, the difficulty of TMT is the treatment course of up to 6 months and the completion rates of usually about 60% only for compliance reason (93), although others reported about completion rates ranging from 38-94% for adjuvant hemithoracic radiation after chemotherapy and surgery [reviewed in Zauderer et al. 2011 (94)]. It has been shown feasible to deliver radiotherapy doses of >45 Gy with both 3D conformal and intensity modulated radiotherapy (IMRT) in the EPP setting (3). IMRT was implemented in a new protocol to be performed also after P/D by the Memorial Sloan Kettering Cancer Center Group in NY. Application of a median dose of 46.8 Gy was associated with grade 3 or higher pneumonitis in 20% of the case, though all but one patient recovered from this event (95), and the median survival was 26 months. Currently this concept is further evaluated in a multicenter phase II toxicity study of chemotherapy (4 cycles cisplatin/pemetrexed +/– pleurectomy/decortication followed by IMRT to the pleura, ClinicalTrials.gov Identifier NCT00715611). Prophylactic port site radiotherapy to prevent mesothelioma seeding through the port sites which has been reported in up to 51% [reviewed in van Zandwijk et al. 2013 (96)] has been concluded in two systematic reviews not to influence significantly the disease course (97,98). A particular promising approach is SMART (“Surgery for Mesothelioma After Radiation Therapy”)—a short accelerated high-dose hemithoracic IMRT followed by EPP recently developed by the Toronto group. Initial results of this phase I/II study showed that the protocol is feasible without elevated perioperative morbidity and mortality and reported promising survival data of 84% cumulative 3-year survival rates in the subgroup of patients with epitheloid histotype (99), but further results with a higher number of patients are awaited (ClinicalTrials.gov Identifier NCT00797719). Finally, RT alone may be used as a palliative treatment for pain control in patients with unresectable tumor.
Attractive treatment approaches to consolidate local tumor control after MCR are localized intracavitary therapies. As aggressive local tumor infiltration into the chest wall and other surrounding tissue is a typical characteristic of MPM, maximal cytoreduction with free margins (R0) can rarely be achieved. This is one of the reasons why local recurrence is a frequent problem in MPM patients after EPP (33%), but even higher after P/D (65%) (37). Any additional treatment to secure these margins is highly desirable and has been explored in many different approaches such as intracavitary chemotherapy, immunotherapy or photodynamic therapy. The advantage of intracavitary chemotherapy therapy is to enable the delivery of higher local doses to surgical margins with less toxicity than systemic therapy; this pharmacokinetic advantage has been proven (100,101). Hyperthermia has been utilized to increase absorption and cytotoxicity. Heated intraoperative chemotherapy lavage (HIOC) after MCR (EPP or P/D) has been studied extensively in several phase II clinical trials by the Sugarbaker group (102,103) and recently they reported extended interval to recurrence (27.1 vs. 12.8 months) and overall survival (35.3 vs. 22.8 months) in a group of low-risk patients with epithelioid histopathology and other favorable prognostic factors compared with controls (104). However, renal toxicity, is a significant dose-limiting concern, but has been decreased by the use of renal cytoprotectants (105). The pharmacokinetic advantage of localized therapy might be further improved by binding cytotoxic (or other agents) to a fibrin carrier—a concept which has been studied in several preclinical models (106-109). Currently the concept of localized intracavitary Cisplatin-Fibrin chemotherapy after P/D or EPP is evaluated in a phase I Dose-Escalation/Phase IIa trial to assess safety and toxicity of the treatment (NCT01644994 Influence Meso).
Another localized treatment approach is intracavitary photodynamic therapy, a light-based treatment consisting of three compounds: a nontoxic photosensitizing compound, oxygen and visible light. It is an FDA approved treatment and can be also performed after lung-sparing P/D. Pleural photodynamic therapy was first explored for MPM by Pass et al. in the 1990 (110) and since then further elaborated by several groups, but most intensively by Friedberg and colleagues. Besides very promising survival data, the Philadelphia group, have found an additional immunologic effect by rendering cancer cells more presentable to the immune system after PDT (111).
Summary
Best survival data in patients with MPM are currently reported from groups using multimodality treatment including MCR achieved either by EPP or eP/D for patients qualifying as far as tumor stage and functional reserve are concerned. In general, several treatment combinations have been applied ranging from systemic (neo- or adjuvant) to localized chemotherapy, neo- or adjuvant radiotherapy and others. The choice of the surgical procedure should be tailored according to tumor stage, performance status, and institutional experience. Morbidity and mortality of these treatment approaches have been reduced at experienced centres indicating that this complex treatment should be performed at dedicated high volume mesothelioma centers.
Acknowledgements
I want to thank Prof. Dr. Walter Weder for critical reading of the present article. Also I want to thank our research team Dr. Karima Berard, Dr. Martina Friess, Dr. Mayura Meerang, Dr. Chloe Spichiger and Guillaume Wuilleret for their ongoing good team work in mesothelioma research and the team of the Division of Thoracic Surgery—Director Professor Walter Weder—and all the other disciplines involved for the treatment of MPM patients, Institute of Diagnostic Radiology (Dr. Thomas Frauenfelder, Dr. Thi Dan Linh Nguyen-Kim), Institute of Surgical Pathology (Prof. Dr. Alex Soltermann, Dr. Bart Vrugt, Prof. Dr. Holger Moch) Laboratory of Molecular Oncology (Dr. Alessandra Curioni, Dr. Emanuela Felley-Bosco, Prof. Dr. Rolf Stahel), and for good collaboration.
Disclosure: The author declares no conflict of interest.
References
- Park EK, Takahashi K, Hoshuyama T, et al. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect 2011;119:514-8. [PubMed]
- Lianes P, Remon J, Bover I, et al. SEOM guidelines for the treatment of malignant pleural mesothelioma. Clin Transl Oncol 2011;13:569-73. [PubMed]
- Stahel RA, Weder W, Lievens Y, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v126-8. [PubMed]
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260-71. [PubMed]
- Marinaccio A, Binazzi A, Cauzillo G, et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur J Cancer 2007;43:2722-8. [PubMed]
- Peto J, Decarli A, La Vecchia C, et al. The European mesothelioma epidemic. Br J Cancer 1999;79:666-72. [PubMed]
- Patz EF Jr, Shaffer K, Piwnica-Worms DR, et al. Malignant pleural mesothelioma: value of CT and MR imaging in predicting resectability. AJR Am J Roentgenol 1992;159:961-6. [PubMed]
- Eibel R, Tuengerthal S, Schoenberg SO. The role of new imaging techniques in diagnosis and staging of malignant pleural mesothelioma. Curr Opin Oncol 2003;15:131-8. [PubMed]
- Wang ZJ, Reddy GP, Gotway MB, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. Radiographics 2004;24:105-19. [PubMed]
- Gill RR. Imaging of mesothelioma. Recent Results Cancer Res 2011;189:27-43. [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [PubMed]
- Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004;15:257-60. [PubMed]
- Armato SG 3rd, Ogarek JL, Starkey A, et al. Variability in mesothelioma tumor response classification. AJR Am J Roentgenol 2006;186:1000-6. [PubMed]
- Frauenfelder T, Tutic M, Weder W, et al. Volumetry: an alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J 2011;38:162-8. [PubMed]
- Ceresoli GL, Chiti A, Zucali PA, et al. Early response evaluation in malignant pleural mesothelioma by positron emission tomography with [18F]fluorodeoxyglucose. J Clin Oncol 2006;24:4587-93. [PubMed]
- Travis W, Brambilla E, Muller-Hermelink H, et al. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus, and Heart. World Health Organization Classification of Tumours. Lyon, France: IARC Press, 2004.
- Van Schil PE, Opitz I, Weder W, et al. Multimodal management of malignant pleural mesothelioma: where are we today? Eur Respir J 2014. [Epub ahead of print]. [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [PubMed]
- Mattson K. Natural history and clinical stage of malignant pleural mesothelioma. Eur J Respir Dis 1982;63:87.
- Sugarbaker DJ, Norberto JJ, Swanson SJ. Surgical staging and work-up of patients with diffuse malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 1997;9:356-60. [PubMed]
- Speissel B, Beahrs O, Hermanek P. International Union Against Cancer (UICC): TNM Atlas: Springer: Berlin, 1992.
- AJCC Manual for staging of cancer. 4th ed. Philadelphia: J.B. Lipincott, 1992.
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995;108:1122-8. [PubMed]
- Rusch VW, Giroux D. Do we need a revised staging system for malignant pleural mesothelioma? Analysis of the IASLC database. Ann Cardiothorac Surg 2012;1:438-48. [PubMed]
- Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138-46. [PubMed]
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304-12. [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [PubMed]
- Branscheid D, Krysa S, Bauer E, et al. Diagnostic and therapeutic strategy in malignant pleural mesothelioma. Eur J Cardiothorac Surg 1991;5:466-72; discussion 473. [PubMed]
- Allen KB, Faber LP, Warren WH. Malignant pleural mesothelioma. Extrapleural pneumonectomy and pleurectomy. Chest Surg Clin N Am 1994;4:113-26. [PubMed]
- Baldini EH, Recht A, Strauss GM, et al. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 1997;63:334-8. [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [PubMed]
- Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999;68:1799-804. [PubMed]
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95. [PubMed]
- Aziz T, Jilaihawi A, Prakash D. The management of malignant pleural mesothelioma; single centre experience in 10 years. Eur J Cardiothorac Surg 2002;22:298-305. [PubMed]
- Pagan V, Ceron L, Paccagnella A, et al. 5-year prospective results of trimodality treatment for malignant pleural mesothelioma. J Cardiovasc Surg (Torino) 2006;47:595-601. [PubMed]
- Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257-64; discussion 264. [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620. [PubMed]
- Luckraz H, Rahman M, Patel N, et al. Three decades of experience in the surgical multi-modality management of pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:552-6. [PubMed]
- Tonoli S, Vitali P, Scotti V, et al. Adjuvant radiotherapy after extrapleural pneumonectomy for mesothelioma. Prospective analysis of a multi-institutional series. Radiother Oncol 2011;101:311-5. [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: A harmful procedure. Lung Cancer 2012;77:151-5. [PubMed]
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202. [PubMed]
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8. [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [PubMed]
- Buduhan G, Menon S, Aye R, et al. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 2009;88:870-5; discussion 876. [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [PubMed]
- Federico R, Adolfo F, Giuseppe M, et al. Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by surgery and radiation in the treatment of pleural mesothelioma. BMC Cancer 2013;13:22. [PubMed]
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 4-5. [PubMed]
- Cao CQ, Yan TD, Bannon PG, et al. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol 2010;5:1692-703. [PubMed]
- Achatzy R, Beba W, Ritschler R, et al. The diagnosis, therapy and prognosis of diffuse malignant mesothelioma. Eur J Cardiothorac Surg 1989;3:445-7; discussion 448. [PubMed]
- Ruffie P, Feld R, Minkin S, et al. Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: a retrospective study of 332 patients. J Clin Oncol 1989;7:1157-68. [PubMed]
- Pass HI, Kranda K, Temeck BK, et al. Surgically debulked malignant pleural mesothelioma: results and prognostic factors. Ann Surg Oncol 1997;4:215-22. [PubMed]
- Nakas A, Martin Ucar AE, Edwards JG, et al. The role of video assisted thoracoscopic pleurectomy/decortication in the therapeutic management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008;33:83-8. [PubMed]
- Nakas A, von Meyenfeldt E, Lau K, et al. Long-term survival after lung-sparing total pleurectomy for locally advanced (International Mesothelioma Interest Group Stage T3-T4) non-sarcomatoid malignant pleural mesothelioma. Eur J Cardiothorac Surg 2012;41:1031-6. [PubMed]
- Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 2012;93:1658-65; discussion 1665-7.
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. [PubMed]
- Cao C, Krog Andvik SK, et al. Staging of patients after extrapleural pneumonectomy for malignant pleural mesothelioma--institutional review and current update. Interact Cardiovasc Thorac Surg 2011;12:754-7. [PubMed]
- Ambrogi V, Mineo D, Gatti A, et al. Symptomatic and quality of life changes after extrapleural pneumonectomy for malignant pleural mesothelioma. J Surg Oncol 2009;100:199-204. [PubMed]
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9. [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [PubMed]
- Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:149-53. [PubMed]
- Waller DA, Morritt GN, Forty J. Video-assisted thoracoscopic pleurectomy in the management of malignant pleural effusion. Chest 1995;107:1454-6. [PubMed]
- Halstead JC, Lim E, Venkateswaran RM, et al. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur J Surg Oncol 2005;31:314-20. [PubMed]
- Martin-Ucar AE, Edwards JG, Rengajaran A, et al. Palliative surgical debulking in malignant mesothelioma. Predictors of survival and symptom control. Eur J Cardiothorac Surg 2001;20:1117-21. [PubMed]
- Nakas A, Trousse DS, Martin-Ucar AE, et al. Open lung-sparing surgery for malignant pleural mesothelioma: the benefits of a radical approach within multimodality therapy. Eur J Cardiothorac Surg 2008;34:886-91. [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63: discussion 263-4.
- Hunt BM, Farivar AS, Vallieres E, et al. Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg 2012;94:1053-7; discussion 1057-9. [PubMed]
- Rintoul RC, Ritchie AJ, Edwards J, et al. Mesovats: a multi-centre randomised controlled trial of video assisted thoracoscopic pleurectomy versus talc pleurodesis in malignant pleural mesothelioma. J Thorac Oncol 2013;8:2-3.
- Ettinger DS, Akerley W, Borghaei H, et al. Malignant pleural mesothelioma. J Natl Compr Canc Netw 2012;10:26-41. [PubMed]
- Allen AM, Czerminska M, Janne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 2006;65:640-5. [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. [PubMed]
- Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1998;115:310-7; discussion 317-8. [PubMed]
- Gill RR, Richards WG, Yeap BY, et al. Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: stratification of survival with CT-derived tumor volume. AJR Am J Roentgenol 2012;198:359-63. [PubMed]
- Campbell NP, Kindler HL. Update on malignant pleural mesothelioma. Semin Respir Crit Care Med 2011;32:102-10. [PubMed]
- Hollevoet K, Nackaerts K, Thimpont J, et al. Diagnostic performance of soluble mesothelin and megakaryocyte potentiating factor in mesothelioma. Am J Respir Crit Care Med 2010;181:620-5. [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2003;21:2636-44. [PubMed]
- Merritt N, Blewett CJ, Miller JD, et al. Survival after conservative (palliative) management of pleural malignant mesothelioma. J Surg Oncol 2001;78:171-4. [PubMed]
- Calavrezos A, Koschel G, Husselmann H, et al. Malignant mesothelioma of the pleura. A prospective therapeutic study of 132 patients from 1981-1985. Klin Wochenschr 1988;66:607-13. [PubMed]
- Byrne MJ, Davidson JA, Musk AW, et al. Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol 1999;17:25-30. [PubMed]
- Castagneto B, Zai S, Dongiovanni D, et al. Cisplatin and gemcitabine in malignant pleural mesothelioma: a phase II study. Am J Clin Oncol 2005;28:223-6. [PubMed]
- Kalmadi SR, Rankin C, Kraut MJ, et al. Gemcitabine and cisplatin in unresectable malignant mesothelioma of the pleura: a phase II study of the Southwest Oncology Group (SWOG 9810). Lung Cancer 2008;60:259-63. [PubMed]
- Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 2002;87:491-6. [PubMed]
- Karrison T, Kindler HL, Gandara DR. Final analysis of a multi-center, double-blinded, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin (GC) plus bevacizumab (B) or placebo (p) in patients (pts) with malignant mesothelioma (MM). ASCO 2007.
- van Haarst JM, Baas P, Manegold C, et al. Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer 2002;86:342-5. [PubMed]
- Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685-94. [PubMed]
- Bitanihirwe BK, Meerang M, Friess M, et al. PI3K/mTOR Signaling in Mesothelioma Patients Treated with Induction Chemotherapy Followed by Extrapleural Pneumonectomy. J Thorac Oncol 2014;9:239-47. [PubMed]
- Cedrés S, Montero MA, Martinez P, et al. Exploratory analysis of activation of PTEN-PI3K pathway and downstream proteins in malignant pleural mesothelioma (MPM). Lung Cancer 2012;77:192-8. [PubMed]
- Bech C, Sorensen JB. Chemotherapy induced pathologic complete response in malignant pleural mesothelioma: a review and case report. J Thorac Oncol 2010;5:735-40. [PubMed]
- Oxnard GR, Armato SG 3rd, Kindler HL. Modeling of mesothelioma growth demonstrates weaknesses of current response criteria. Lung Cancer 2006;52:141-8. [PubMed]
- Righi L, Papotti MG, Ceppi P, et al. Thymidylate Synthase But Not Excision Repair Cross-Complementation Group 1 Tumor Expression Predicts Outcome in Patients With Malignant Pleural Mesothelioma Treated With Pemetrexed-Based Chemotherapy. J Clin Oncol 2010;28:1534-9. [PubMed]
- Gupta V, Krug LM, Laser B, et al. Patterns of Local and Nodal Failure in Malignant Pleural Mesothelioma After Extrapleural Pneumonectomy and Photon-Electron Radiotherapy. J Thorac Oncol 2009;4:746-50. [PubMed]
- Baldini EH. External beam radiation therapy for the treatment of pleural mesothelioma. Thorac Surg Clin 2004;14:543-8. [PubMed]
- Van Schil PE. Follow-up after lung cancer resection: is intensified also justified? Eur Respir J 2013;42:1178-9. [PubMed]
- Zauderer MG, Krug LM. The evolution of multimodality therapy for malignant pleural mesothelioma. Curr Treat Options Oncol 2011;12:163-72. [PubMed]
- Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural Intensity-Modulated Radiotherapy for Malignant Pleural Mesothelioma. Int J Radiat Oncol Biol Phys 2012;83:1278-83. [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254-E307. [PubMed]
- Lee CW, Murray N, Anderson H, et al. Outcomes with first-line platinum-based combination chemotherapy for malignant pleural mesothelioma: a review of practice in British Columbia. Lung Cancer 2009;64:308-13. [PubMed]
- Nagendran M, Pallis A, Patel K, et al. Should all patients who have mesothelioma diagnosed by video-assisted thoracoscopic surgery have their intervention sites irradiated? Interact Cardiovasc Thorac Surg 2011;13:66-9. [PubMed]
- Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: the “SMART” approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014;9:397-402. [PubMed]
- Rusch VW, Niedzwiecki D, Tao Y, et al. Intrapleural cisplatin and mitomycin for malignant mesothelioma following pleurectomy: pharmacokinetic studies. J Clin Oncol 1992;10:1001-6. [PubMed]
- Sugarbaker PH, Stuart OA, Eger C. Pharmacokinetics of Hyperthermic Intrathoracic Chemotherapy following Pleurectomy and Decortication. Gastroenterol Res Pract 2012;2012:471205.
- Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg 2009;138:405-11. [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II Study of Pleurectomy/Decortication and Intraoperative Intracavitary Hyperthermic Cisplatin Lavage for Mesothelioma. J Clin Oncol 2006;24:1561-7. [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [PubMed]
- Mujoomdar AA, Sugarbaker DJ. Hyperthermic chemoperfusion for the treatment of malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2008;20:298-304. [PubMed]
- Opitz I, Lardinois D, Arni S, et al. Local recurrence model of malignant pleural mesothelioma for investigation of intrapleural treatment. Eur J Cardiothorac Surg 2007;31:773-8. [PubMed]
- Opitz I, Erne BV, Demirbas S, et al. Optimized intrapleural cisplatin chemotherapy with a fibrin carrier after extrapleural pneumonectomy: a preclinical study. J Thorac Cardiovasc Surg 2011;141:65-71. [PubMed]
- Lardinois D, Jung FJ, Opitz I, et al. Intrapleural topical application of cisplatin with the surgical carrier Vivostat increases the local drug concentration in an immune-competent rat model with malignant pleuromesothelioma. J Thorac Cardiovasc Surg 2006;131:697-703. [PubMed]
- Ampollini L, Soltermann A, Felley-Bosco E, et al. Immuno-chemotherapy reduces recurrence of malignant pleural mesothelioma: an experimental setting. Eur J Cardiothorac Surg 2009;35:457-62. [PubMed]
- Pass HI, Tochner Z, DeLaney T, et al. Intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 1990;50:687-8. [PubMed]
- Friedberg JS. Photodynamic therapy as an innovative treatment for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2009;21:177-87. [PubMed]