Salvage esophagectomy
Definitions
Planned resection: esophagectomy that occurs after preoperative therapy, usually within three months of therapy.
Salvage resection: surgical therapy after local-regional recurrence.
Observation: an active process of patient re-evaluation after definitive medical therapy. Optimally this involves PET imaging and EGD with endoscopic ultrasound at q4 monthly basis for the initial two years after therapy.
Introduction
Salvage esophagectomy can be defined in different ways, such as time elapsed from therapy or intent to treat. Patients with esophageal cancer who were treated with chemoradiation as definitive therapy (dCXRT) but are later identified as having local-regional recurrence or persistent disease and are resected satisfy the definition of salvage. Other definitions are included in the literature, such as patients who underwent resection in a delayed manner after induction chemoradiation, usually with a 3-month or greater interval from completion of therapy to resection. It is important to understand the basic definitions to interpret the available data. Patients treated with definitive intent will have avoided surgery by design; thus, when recurrence is detected, the decision to proceed to resection is dependent on the knowledge of disease, rather than patient fitness. On the other hand, patients who were delayed from undergoing a planned resection because of poor performance status after completion of chemoradiation (CXRT), and who later were identified as having persistent disease and sufficient performance improvement to tolerate a resection represents a different patient population with different risks and outcomes.
Thus, patients arrive at dCXRT by different routes. Decreased performance after preoperative CXRT can result in a “watch and wait” strategy rather than proceeding to the planned resection. This is a method of backing in to definitive therapy by risk/necessity. If a patient is not cured by the initial therapy, recurrence noted during a period of observation should stimulate a discussion for salvage surgery. One very important caveat is that the method and frequency of observation in patients treated with this strategy effects the surgeon’s ability to offer resection. Local-regional recurrences that go unrecognized will progress to a point of non-resectability even in the situation where there is no evidence of distant disease. From the standpoint of clinicians, this should be recognized as the ultimate failure of therapy; death from local-regional disease.
The optimal management of locally advanced cancer of the esophagus remains controversial. Published results of pathologic complete response after combined concurrent chemoradiation therapies for esophageal cancer have caused some medical and radiation oncologists to question the additional benefit of surgical resection in patients that respond to non-surgical therapy. To that end, patients may not ever see a surgeon to consider the option of resection until after there is a recurrence, and sometimes not until the recurrence has failed multiple attempts at systemic or combined non-operative therapy.
Finally, to be complete we should clarify the definition of selective surgical approach. In this strategy, patients treated with CXRT to a complete response would be observed, those in whom there is residual disease, as judged by the med/surgical team, would proceed to planned resection. All of these pathways to salvage resection may result in subtle or even significant differences in patients and potential risk/benefit ratio for surgery. These differences need to be carefully considered prior to embarking on a physician-patient discussion about surgery.
Definitive chemoradiation therapy
When patients present with what appears clinically to be locally advanced disease on staging work up, they actually have a variety of potential outcomes. The biologic heterogeneity of esophageal cancer and lack of accurate staging technologies results in an inability to recognize patients with systemic disease versus those who are curable by local-regional treatment modalities. It has been assumed that resection in this group of patient’s results in discouraging long-term outcomes, primarily as a result of an inability to predict who will ultimately die of systemic disease. Optimally, we would offer surgery to only patients that would benefit, without omitting patients who need surgery, and every patient who underwent resection would have an excellent outcome. But, as long as esophagectomy is regarded as an operation carrying significant potential for morbidity, mortality, and changes in quality of life, patients who are either incapable or unwilling to undergo resection will opt to be treated medically.
This is precisely what occurred. Clinicians treating patients who were omitted from surgical therapy found some limited success with innovative combined therapies (chemotherapy and concurrent radiation). This led to a paradigm shift; the inevitable conclusion that patients with better performance status and potentially curable disease may also perform well with medical therapy as an alternative to resection (1). In fact, there are a number of phase II and III trials illustrating the potential of non-surgical therapy to produce short and long-term survival (Table 1) (1-10). One landmark study of medical-only therapy in a cohort of potentially curable patients describes the long-term results of patients treated with chemotherapy with concurrent radiation versus radiation alone. This study by the Radiation Therapy Oncology Group (RTOG 85-01/INT 0123) reported median and 5-year survival of 14.1 months and 27% in the group treated with definitive chemotherapy and radiation (6). In follow up to INT 0123 was a publication of mature data from both the randomized cohort and an additional group of non-randomized patients, the majority of who had squamous cell carcinoma (2). The authors reported 5-year survival between 14-26% in the non-randomized and randomized patient cohorts respectively. The ability to achieve prolonged survival with medical therapy was somewhat encouraging in the randomized group, but intermediate to poor in the non-randomized group.

Full table
Most significant was number of patients that failed local-regionally in that trial: 56%. The RTOG 94-05 trial was designed to address this issue by utilizing higher levels of radiation, presumably to help sterilize the local-regional tumor fields (3). The results were disappointing; this trial illustrated that higher radiation levels, in the manner in which they were dosed in this trial, failed to improve local-regional control or survival, and therefore the 50.4 Gy dose of radiation described in the original INT-0123 trial became the standard for definitive dose for radiation for thoracic esophageal carcinoma treated with concurrent chemotherapy. This dose has since been adopted by many centers as the standard for preoperative chemoradiation therapy as well. The advantage being that a selective approach to surgery could be employed should that become the more attractive treatment option.
There was further evidence in surgical series illustrating that with modern chemoradiation protocols, patients treated with multi-modality therapy reached a pathologic complete response frequently (20-40%) (2-5,7-9). Given this response there were clinician groups that were of the opinion that surgery was merely documenting the response to therapy rather than complementing the outcome (11). The ensuing controversy led to an opinion shift in treatment approach. Rather than seeking to improve upon surgical outcomes with pre or post-operative therapy, the question arose: what is the additional benefit of esophagectomy in patients who have responded to definitive chemoradiation?
Chemoradiation with or without surgery
There are two randomized controlled trials comparing the benefit of adding surgery to definitive chemoradiation therapy (4,5). Both trials primarily involve squamous cell carcinoma of the esophagus. The study by Bedenne et al. randomized patients that responded to chemoradiation either to a surgery or observation arm. Two hundred fifty patients were evaluated (129 surgical, 130 definitive chemoradiation therapy; 11% adenocarcinoma). Median survival in the surgical and non-surgical groups was 17.7 months versus 19.3 months, respectively, and 2-year overall survival was 34% versus 40%, respectively (P= NS). There were notable benefits found in the surgical arm such as improved local-regional control and increased freedom from palliative procedures (such as stents), but the trade-off was significantly higher treatment related toxicity in the surgical arm. Mortality analyzed at 90 days was 9.3% in the surgical group versus 0.8% in the non-surgical group (4). The fact that improved local control in the surgical arm did not lead to an increase in overall survival in this study may exemplify the difficulty with adequately staging patients pretreatment and the biologic heterogeneity that is inherent with esophageal cancer. Further, the data exemplifies that esophagectomy after concurrent chemoradiation in a multi-institutional setting can lead to higher than expected mortality which will decrease the value of resection.
The study by Stahl et al. employed induction chemotherapy prior to chemoradiation presumably in an effort to decrease distant failure (5). All patients in this trial had squamous cell carcinoma. This study randomized 172 patients (86 to chemoradiation followed by surgery versus 86 treated with definitive chemoradiation). The results showed freedom from local-regional recurrence was better with surgery and disease-free survival was reported to be significantly improved with surgery compared to observation (64% versus 41% at 2 years; P=0.003). However in contrast to the Bedenne trial (4), the Stahl trial (which randomized all patients rather than responders only) did demonstrate a survival advantage in the surgery arm (31% versus 24% at 3 years; P=0.02). Again, there was a significant increase in treatment-related mortality reported in the surgical arm (12.8% versus 3.5%; P=0.03). Overall this study illustrated that patients who underwent surgery were less likely to die of cancer but were at increased risk for treatment-related toxicity. Another finding of interest on sub-group analysis was that non-responders who achieved a complete (R0) resection reached 32% three-year survival. This was in contrast to responders who achieved greater than 50% three-year survival regardless of the treatment arm.
Taken together, these two studies demonstrate that in patients who respond to medical therapy, the risk of increased toxicity seen in multi-institutional trials involving combined modality therapy including surgery may detract from a potential advantage in disease-free survival obtained by the addition of surgery. A second observation is that non-responders may derive more benefit from surgery than responders.
Salvage esophagectomy
Definitive chemoradiation therapy as a treatment strategy has created a unique subgroup of patients who eventually manifest regrowth of residual viable tumor or re-present with recurrence in a local-regional distribution in the absence of metastatic disease. These patients face limited treatment alternatives that can lead to cure, and should absolutely be evaluated by an esophageal surgeon to discuss the option of salvage esophagectomy. Other methods of therapy such as retreatment with chemoradiation may be possible for previously untreated regional disease, but tumor that regrows within the radiation field after medical therapy is resistant and unlikely to respond well to retreatment.
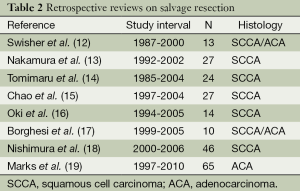
There are many prospective, non-randomized and retrospective publications describing the feasibility of salvage esophagectomy (Table 2). Whereas most focus on squamous cell carcinoma, there is also published experience on salvage resection for adenocarcinoma (12-24). Most of the published data are small retrospective series ranging from 10 to 65 patients. A comprehensive review by Gardner-Thorpe summarizes nine published series totaling 105 patients (Table 3) (27). The indication for salvage in over 50% of the resected cases was for persistent disease, while local-regional recurrence in the absence of metastatic disease was the indication in 43%. One of the interesting questions about definitive CXRT is the percentage of patients that will ultimately require surgery, and how many will have missed an opportunity for cure because planned surgery was avoided. Based on available data the absolute percentage of patients that will present for salvage resection after definitive medical therapy is not known. Selection for salvage resection depends on many factors such as initial stage, indications for resection, patient demographics, referral patterns, etc. However, there is one report by Nishimura et al. that reported on 16% of the thoracic esophageal cancer patients that underwent definitive CXRT at their institution who were referred for salvage resection (18).
Full table

Full table
Regarding surveillance for patients undergoing observation after definitive CXRT, patients whose recurrence is discovered because of symptoms are generally further advanced than those that are discovered by imaging or endoscopy. Often this means that the patient will not be amenable to salvage resection. In contrast, patients who are followed closely with imaging and endoscopy are probably (observational data from author) more likely to have recurrence detected at an early enough stage to potentially benefit from salvage resection. Therefore, it is recommended that patients undergo endoscopy with ultrasound (to detect nodal disease, not wall thickness) along with PET imaging every four months during the first 1-2 years after CXRT, followed by surveillance at 6-12 months intervals thereafter. Up to 95% of patients will recur within two years of definitive CXRT, and almost all within three years (99%) (25).
Patient selection
Patients who present persistent or recurrent local-regional disease after definitive CXRT and have no evidence of systemic disease are candidates for salvage resection. Similarly, for a patient whose surgery was cancelled due to a decline in performance status but has since improved, recognition of disease should prompt a re-evaluation for surgery. A re-staging work up should be performed prior to considering salvage resection, and this would include methods to rule out systemic disease such as high definition CT to rule out metastatic disease in the lungs and integrated PET/CT for the whole body. Endoscopy is used to assess the proximal and distal extent of tumor involvement for resection and reconstruction planning. Endoscopic ultrasound is not reliable for assessing esophageal wall invasion after radiation treatment but can be very helpful when combined with transesophageal or transbronchial fine needle aspiration to assess regional and non-regional nodes of interest. These low risk diagnostic methods can offer histologic confirmation of disease where there is question of non-regional lymph node or adrenal gland involvement for example, that would preclude the indication for salvage resection. Bronchoscopy is necessary in patients with proximal tumors above or around the carina when there is suspicion for direct invasion into the airway. Endobronchial ultrasound (EBUS) can be helpful in this situation as well. A physiologic work up consisting of serum laboratory analyses, cardiac and pulmonary evaluations should be considered prior to resection. In patients where elevated CEA was a marker for disease, drawing baseline levels can be helpful for later surveillance.
Surgical resection
Reported series have consisted of resections performed with transhiatal, McKeown and Ivor Lewis approaches (12-28). There is no literature supporting a limited lymphadenectomy for salvage resection and therefore we advocate a complete resection with a two-field lymph node dissection when possible. However, one review does describe significantly fewer 3-field lymphadenectomies performed for salvage compared to planned esophagectomy (41% vs. 91%) (20). Two-field lymphadenectomy has been described, and there is no direct evidence that this contributes to significant morbidity (12). We perform salvage resection exactly as a standard esophagectomy (including the use of minimally invasive techniques) with some caveats. Alternative methods of reconstruction and the possibility for resection of primary tumor with a second, staged reconstruction effort at a later date either due to poor patient performance or for lack of an appropriate conduit should be considered (14).
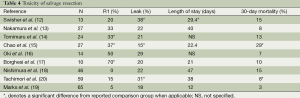
One of the more striking morbidities that have been reported in multiple series describing salvage esophagectomy is the rate of conduit necrosis, quoted as high as 25% (13,28). Although ours is the largest series on salvage for adenocarcinoma (65 patients) our rate of conduit loss is still a bit higher than our historical data and in comparison to planned resection (4.6% compared to 1%) (19). Most surgeons would agree that the stomach is the most robust and straight-forward esophageal replacement, however, for patients with lower esophageal tumors review of the radiation treatment plan will often reveal inclusion of the entire stomach within the treatment field, usually to full dose. The latent period between the completion of radiation and the surgical resection may affect the extent of small-vessel radiation damage and potentially jeopardize the viability of the stomach when transposed into the chest. In these situations we make a practice of carefully examining the stomach intra-operatively for signs of damage or suitability as a reconstruction conduit. Similarly, fashioning the anastomosis within the radiated field in the chest has been shown by our group to result in a higher than acceptable leak rate (29). When the potential viability of the stomach is in question, one or more of several responses should be prompted: harvest of omentum to transpose into the chest to wrap the gastric and anastomotic suture/staple lines (30), consideration for use of a different conduit such as a colon or long segment jejunal interposition with microvascular augmentation, or esophageal resection with delayed reconstruction. We recommend that the esophageal anastomosis be place above the previous radiation field when at all possible. Another potential drawback to salvage resection is the potential for incomplete resection. Available data reports that 10-70% of resections performed in a salvage situation are R1 or R2 (12-14,17,19). In our series 91% (59/65) of patients had an R0 resection (19).
Toxicity
Another barrier to adopting salvage resection as the primary treatment modality for thoracic esophageal cancer is the described toxicity associated with salvage resection. This is summarized in Table 4. In-hospital deaths range from 2% to 33% (12-28); the upper range being significantly higher than optimal. In some series hospital stays were longer in general (14 to 47 days) and this may be due to an increased incidence of conduit necrosis, pulmonary toxicities and/or anastomotic leak. As previously mentioned, conduit necrosis, when described, was seen in up to 25% and anastomotic leak in 15% to 39% of patients undergoing salvage resection. In fact, many of the perioperative deaths described in these series were related to anastomotic leak or conduit necrosis despite aggressive medical and surgical efforts to rescue these patients. Other reported discrepancies from standard, planned resection include potential for more blood transfusion and ICU stay. In order to explore the potential reasons for any differences in our series we performed a matched pair analysis between a planned and salvage resection cohort. Those results showed that salvage resection was not the predictor of complication above and beyond co-morbidity (controlled for in matched pair) and disease stage. Variables such as length of stay, ICU admission, OR time, blood loss and leak rate were comparable. With careful selection patients can achieve an excellent outcome in experienced centers (19).
Full table
Outcome
Patients who have undergone salvage resection represent a highly selected group of patients with potentially favorable biology. Less fortunate patients with poor performance status, progressive systemic disease, or unresectable local-regional recurrence are, by definition, eliminated from this group thus improving the appearance of the overall outcome for this selected group. With this in mind, the reported 5-year overall survival is up to 60% in patients who were fortunate enough to undergo an R0 resection (12). However, as a group that includes R0-1 resections long term survival is intermediate, ranging from 0-35% at five years (12-24,27).
The data for patients with adenocarcinoma undergoing salvage resection is similar to squamous histology. We presented a series describing salvage for exclusively adenocarcinoma; 65 patients who presented for resection after definitive chemoradiation for adenocarcinoma of the esophagus achieved a 32% overall 5-year survival. The median survival was not statistically different from a comparison group of 521 patients who underwent planned resection (48 versus 32 months, P=0.22) (19).
Selective surgery
Trials comparing chemoradiation with or without surgery have illustrated that cure is possible without surgical resection. Choosing the patient that requires further local-regional therapy after chemoradiation as opposed to one who would not benefit from resection compels the possibility of selective surgical resection; incomplete responders would go for surgery and patients that are clinical complete responders or progressed to distant disease on therapy would avoid resection. There have been two recent prospective, nonrandomized trials that sought to evaluate a selective surgical approach as an adjunct to definitive medical therapy in patients with squamous histology (Table 5) (31,32). Both show high clinical response rates to therapy suggesting that a selective surgical approach using chemoradiotherapy represents a survival advantage over surgery alone. Similarly, a phase II study including patients with adenocarcinoma was completed by the RTOG (protocol 0246) showing feasibility of this approach in a multi-institutional study albeit with high medical mortality (5/41) (33). None of these studies were designed to show superiority over a planned tri-modality chemoradiotherapy + surgery approach and therefore conclusions cannot be reached on that question. What the RTOG 0246 study did show very nicely is that among the patients taken to selective resection, when the surgeon suspected that there would be persistent disease the results showed that 17/18 patients had disease in the pathologic specimen. The only patient in the study that was resected to a pathologic complete response had insisted on surgical therapy. These results underscore the positive predictive value of surgeons who are experienced in multi-modality therapy to predict when viable tumor will be present in the specimen. Several patients in that series also presented for later salvage resection, indicating that accuracy is not complete.

Full table
Based on the literature, it is reasonable to conclude that patients with locally advanced esophageal adenocarcinoma have better outcomes with planned resection compared to observation, as long as they are candidates for trimodality therapy (CXRT + surgery) (34).
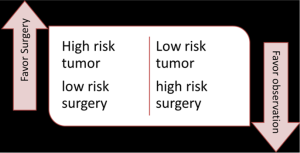
As well, when considering selective surgery after successful chemoradiation, one should stratify patients by risk of surgery balanced with risk of recurrence (Figure 1). High-risk tumor, low-risk patients should prompt a planned resection. Lower-risk tumor (based on initial stage and response) in a high-risk patient will encourage me to consider selective surgery—observation (35).
Summary pearls
- Patients who have received dCXRT should have active surveillance for early detection of recurrence.
- All medically fit patients with local regional recurrence after dCXRT should be referred to a surgeon to consider resection.
- Patient selection for salvage is essential. Staging for metastatic disease and physiologic work up should be complete.
- Patients should be referred to centers experienced in multi-modality treatment of esophageal cancer and salvage resection.
- Reviewing the previous radiation treatment plan is essential.
- Anastomoses should be placed above of the esophageal radiation field.
- Alternative conduits may be appropriate.
In conclusion, salvage resection is a reasonable option to treat patients with local regional recurrence after failed definitive therapy.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Leichman L, Herskovic A, Leichman CG, et al. Nonoperative therapy for squamous-cell cancer of the esophagus. J Clin Oncol 1987;5:365-70. [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esoph-ageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Ra-diation Therapy Oncology Group. JAMA 1999;281:1623-7. [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Thorac Oncol 2007;25:1160-8. [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in pa-tients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [PubMed]
- al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiothera-py versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol 1997;15:277-84. [PubMed]
- Ajani JA, Winter K, Komaki R, et al. Phase II randomized trial of two nonoperative reg-imens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol 2008;26:4551-6. [PubMed]
- Algan O, Coia LR, Keller SM, et al. Management of adenocarcinoma of the esophagus with chemoradiation alone or chemoradiation followed by esophagectomy: results of se-quential nonrandomized phase II studies. Int J Radiat Oncol Biol Phys 1995;32:753-61. [PubMed]
- Adams R, Morgan M, Mukherjee S, et al. A prospective comparison of multidisciplinary treatment of oesophageal cancer with curative intent in a UK cancer network. Eur J Surg Oncol 2007;33:307-13. [PubMed]
- Morgan MA, Lewis WG, Casbard A, et al. Stage-for-stage comparison of definitive chemoradiotherapy, surgery alone and neoadjuvant chemotherapy for oesophageal carci-noma. Br J Surg 2009;96:1300-7. [PubMed]
- Hennequin C, Quero L, Baruch-Hennequin V, et al. Do locally advanced esophageal cancer still need surgery? Cancer Radiother 2008;12:831-6. [PubMed]
- Swisher SG, Wynn P, Putnam JB, et al. Salvage esophagectomy for recurrent tumors af-ter definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg 2002;123:175-83. [PubMed]
- Nakamura T, Hayashi K, Ota M, et al. Salvage esophagectomy after definitive chemo-therapy and radiotherapyfor advanced esophageal cancer. Am J Surg 2004;188:261-6. [PubMed]
- Tomimaru Y, Yano M, Takachi K, et al. Factors affecting the prognosis of patients with esophageal cancer undergoing salvage surgery after definitive chemoradiotherapy. J Surg Oncol 2006;93:422-8. [PubMed]
- Chao YK, Chan SC, Chang HK, et al. Salvage surgery after failed chemoradiotherapy in squamous cell carcinoma of the esophagus. Eur J Surg Oncol 2009;35:289-94. [PubMed]
- Oki E, Morita M, Kakeji Y, et al. Salvage esophagectomy after definitive chemoradio-therapy for esophageal cancer. Dis Esophagus 2007;20:301-4. [PubMed]
- Borghesi S, Hawkins MA, Tait D. Oesophagectomy after definitive chemoradiation in patients with locally advanced oesophageal cancer. Clin Oncol (R Coll Radiol) 2008;20:221-6. [PubMed]
- Nishimura M, Daiko H, Yoshida J, et al. Salvage esophagectomy following definitive chemoradiotherapy. Gen Thorac Cardiovasc Surg 2007;55:461-4; discussion 464-5. [PubMed]
- Marks JL, Hofstetter W, Correa AM, et al. Salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma. Ann Thorac Surg 2012;94:1126-32; discussion 1132-3. [PubMed]
- Tachimori Y, Kanamori N, Uemura N, et al. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2009;137:49-54. [PubMed]
- Ishikura S, Nihei K, Ohtsu A, et al. Long-term toxicity after definitive chemoradiothera-py for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 2003;21:2697-702. [PubMed]
- Hironaka S, Ohtsu A, Boku N, et al. Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T(2-3)N(any) M(0) squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2003;57:425-33. [PubMed]
- Meunier B, Raoul J, Le Prisé E, et al. Salvage esophagectomy after unsuccessful curative chemoradiotherapy for squamous cell cancer of the esophagus. Dig Surg 1998;15:224-6. [PubMed]
- Murakami M, Kuroda Y, Okamoto Y, et al. Neoadjuvant concurrent chemoradiotherapy followed by definitive high-dose radiotherapy or surgery for operable thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys 1998;40:1049-59. [PubMed]
- Urschel JD, Ashiku S, Thurer R, et al. Salvage or planned esophagectomy after chemo-radiation therapy for locally advanced esophageal cancer--a review. Dis Esophagus 2003;16:60-5. [PubMed]
- Urschel JD, Sellke FW. Complications of salvage esophagectomy. Med Sci Monit 2003;9:RA173-80. [PubMed]
- Gardner-Thorpe J, Hardwick RH, Dwerryhouse SJ. Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg 2007;94:1059-66. [PubMed]
- Amini A, Ajani J, Komaki R, et al. Factors associated with local-regional failure after de-finitive chemoradiation for locally advanced esophageal cancer. Ann Surg Oncol 2014;21:306-14. [PubMed]
- Juloori A, Tucker SL, Komaki R, et al. Influence of preoperative radiation field on post-operative leak rates in esophageal cancer patients after trimodality therapy. J Thorac Oncol 2014;9:534-40. [PubMed]
- Sepesi B, Swisher SG, Walsh GL, et al. Omental reinforcement of the thoracic esoph-agogastric anastomosis: an analysis of leak and reintervention rates in patients undergoing planned and salvage esophagectomy. J Thorac Cardiovasc Surg 2012;144:1146-50. [PubMed]
- Wilson KS, Lim JT. Primary chemo-radiotherapy and selective oesophagectomy for oe-sophageal cancer: goal of cure with organ preservation. Radiother Oncol 2000;54:129-34. [PubMed]
- Ariga H, Nemoto K, Miyazaki S, et al. Prospective comparison of surgery alone and chemoradiotherapy with selective surgery in resectable squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2009;75:348-56. [PubMed]
- Swisher SG, Winter KA, Komaki RU, et al. A Phase II study of a paclitaxel-based chemoradiation regimen with selective surgical salvage for resectable locoregionally ad-vanced esophageal cancer: initial reporting of RTOG 0246. Int J Radiat Oncol Biol Phys 2012;82:1967-72. [PubMed]
- Murphy CC, Correa AM, Ajani JA, et al. Surgery is an essential component of multimo-dality therapy for patients with locally advanced esophageal adenocarcinoma. J Gastrointest Surg 2013;17:1359-69. [PubMed]
- Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers: Clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2011;9:830-87.


