Current management of esophageal cancer
Introduction
The epidemiology of esophageal cancer has radically changed in the last fifty-years in the Western world. Changes in the predominant type of squamous cell carcinoma (SCC) to adenocarcinoma, disparities between different ethnicities, and the exponential increase in incidence rates of adenocarcinoma have established esophageal cancer as a major public health problem requiring urgent attention specifically in North America (1). It ranks sixth among all cancers in mortality (2) and it is one of the least studied and deadliest cancers worldwide because of its extremely aggressive nature and poor survival rate. The overall 5-year relative survival is 17% (1). Reason to explain this poor outcome stands on the fact that esophageal cancer is diagnosed at rather late stage. Overall at the time of presentation, more than 50% of patients have metastatic disease, near 30% have a locally advanced stage and less 20% have a localized stage that can be cured (1-3).
Management of non metastatic esophageal cancer has evolved since the two last decades. With the advanced of CT-scan, development of the endoscopic ultrasound (EUS) and the emergence of FDG-PET, the assessment of the disease has refined year after year. To date, the staging of the disease is of paramount importance and every treatment decisions should routinely be based on multidisciplinary discussion in the tumor board.
Esophagectomy remains the primary treatment for early stage esophageal cancer although its specific role in superficial (T1A) cancers is still under debate since the development of endoscopic mucosal treatment. There is strong evidence to consider that locally advanced cancers should be recommended for a multimodal treatment with a neoadjuvant chemotherapy or a combined chemoradiotherapy (CRT) followed by surgery (4,5). However, there are differences in the perceived role of surgery in achieving local control between Western and Eastern surgeons, leading to the considerable differences in the use of multidisciplinary therapy. For locally advanced SCC or for a part of adenocarcinoma, some oncologists have proposed treating with definitive CRT to avoid the mortality of surgery. In case of persistent or recurrent disease, a salvage esophagectomy is a possible option but this procedure remains associated with higher levels of perioperative morbidity and mortality.
There is a global agreement over the oncological principles of surgery (6). Surgical resection must consist in a radical, complete, R0, en-bloc esophagectomy associated to an extended two-field lymphadenectomy (7-10). Patients requiring surgical treatment of esophageal cancer should be referred to high-volume centers, especially those with established care pathways or enhanced recovery programs to improve outcomes including morbidity, mortality, survival, and quality of life. Despite the debate over what constitutes the best surgical approach (transthoracic versus transhiatal) (10), the current question is if a minimally procedure could reduce the perioperative morbidity and mortality without jeopardizing the oncological results of surgery. Since the 1990’, minimally invasive esophagectomy (MIE) or hybrid operations are being done in up to 30% of procedures internationally. There are consistent data that minimally invasive procedures could decrease the incidence of respiratory complications and decrease length of hospital-stay. At this point, oncologic outcomes appear equivalent between open and minimally invasive procedures, however numerous clinical phase III are ongoing.
Staging and preoperative assessment
Current management of esophageal cancer is mainly based on exhaustive preoperative assessment. The accuracy of the preoperative staging is essential as the decisions of the tumor board regarding the application of multimodal treatment will be directed according to the accuracy and the specifics of the clinical staging assessment. Standardized assessment of a patient being considered for a curative treatment for early or for advanced esophageal cancer includes upper endoscopy, high-resolution contrast CT scan, FDG-PET scan and EUS (6).
CT scan provides useful information regarding longitudinal extension of the tumor especially with the trachea and the aorta (T4B disease). Suspicions of direct invasion of the thoracic aorta or the tracheobronchial tree should be confirmed with MRI scanning and bronchoscopy respectively. FDG-PET scan provides the most accurate information regarding potential metastatic disease. As a result, FDG-PET scan increases the accuracy for occult metastasis as much as 20% over CT scanning alone (11). Moreover FDG-PET is considered as a reliable technique for post-treatment reassessment and to appreciate the response to neoadjuvant therapy (12). However, its specific role in this situation has to be confirmed (13). EUS provides excellent information with respect to depth of invasion (T status), but its ability to discriminate subtle differences in T1 disease, i.e., T1a versus T1b, is less exact (14). The meta-analysis from Young et al. comparing EUS and endoscopic mucosal resection (EMR) staging demonstrated that EUS predicted accurate depth of tumor invasion in only 56% of patients (14). Therefore, especially if endoscopic treatment is contemplated, staging should include EMR, and any indication of submucosal invasion should lead to recommendation for surgical resection in appropriate candidates. Another limit of the EUS is its ability to provide accurate staging after neoadjuvant therapy. In this context EUS is strongly limited due to post-treatment adherence and fibrosis (15). EUS remains the best modality for assessing locoregional lymph node (LN) involvement especially when fine needle aspiration biopsy of suspicious nodes can be selectively applied to provide specific pathologic information and staging (16).
Early stage cancer
Incidence and definitions
Esophageal adenocarcinoma has seen a dramatic increase in Europe and in the United States over the last 20 years, whereas the rates of SCC of the esophagus has remained relatively stable or decreasing in Western countries (1-3). This epidemiologic change is mainly due to the increase of the Barrett’s Esophagus (BE) in the general population. It is currently estimated that 10% of patients with chronic reflux have BE (1-3). Today, incidence of BE in the USA population may be as high as 5.6% (17,18). In cases of patients with high-grade dysplasia (HGD), up to 30% will develop EAC within five years. Endoscopic surveillance of patient with chronic reflux or known to have BE would explain that 20% of all EAC are detected as an early stage (T1) with disease confined to the mucosa or submucosa (17,18). For SCC, clinical stage I disease accounts for only about 20% of all detected esophageal cancers in Japan (19).
Early stage cancer includes T1a and T1b according to the 7th edition of the AJCC (20). The T1a includes HGD and intramucosal cancer limited to the muscularis mucosa. T1b includes cancer invading muscularis mucosa and extending to the submucosa. A more comprehensive subclassification of early esophageal cancer has been proposed with mucosal disease and submucosal disease divided into three categories respectively (m1-3, and sm1-3) based on depth of invasion (21-23).
HGD and intramucosal tumor
In HGD or in T1a cancer (including m1-3 tumor), the risk of LN disease correlates to the depth of involvement of the cancer and to the histological type. For HGD of for intramucosal cancer, a systematic review of the surgical literature, has reported the rates of occult invasive cancer in patients who were undergoing esophagectomy for prophylactic treatment of HGD. The pooled average was 12.7% in the 441 patients who underwent esophagectomy for HGD among 23 studies (24). The rate of LN involvement for HGD and for intramucosal cancer is estimated between 0 to 2%. A large retrospective review of 126 T1 adenocarcinomas, of which 75 were T1a and 51 T1b, revealed N+ disease of 1.3% and 22% respectively (22). Data on superficial SCC have shown that m3 cancer, or disease extending to the muscularis propria has upwards of 6% risk of LN metastasis (21). Additional characteristics which impact the risk of LN involvement include vascular invasion, tumor size, and the degree of tumor differentiation.
Given the low risk of LN involvement in mucosal disease, there is a general agreement of the reliability and of the efficiency of the endoscopic management of early stage esophageal cancer confined to the mucosa (T1a). Endoscopic resection is, therefore, a potentially curative treatment for such lesions. Initially, options included argon beam coagulation, laser, and photodynamic therapy. More recently, EMR, endoscopic submucosal dissection (ESD), radiofrequency ablation (RFA), cryotherapy, and free-hand mucosal resection have been increasingly applied (25). Because current data on what constitutes the best treatment are limited, it seems not possible at the present time to favor a technique compared to another (26). However, there is global agreement that all visible lesions have to be removed by EMR for definitive histopathological staging and to ensure adequacy of resection margins. This agreement stands on the poor accuracy of EUS to discriminate between T1a and T1b. EMR remains the sole technique able to stage the degree of invasion into the esophageal wall. For intramucosal cancer associated to BE, eradication of the metaplasic mucosa must occur to protect against potential lesion development. For BE segments that measure ≤5 cm and harbor HGD or intramucosal cancer, an EMR approach is used. For patients with BE segments >5 cm, all focal lesions have to be resected with EMR or ESD and the remaining flat BE is ablated using RFA to decrease the rate of stricture formation (25).
Submucosal and T2 tumor
In contrast to T1a tumor where LN invasion is uncommon, invasive cancers (T1b and T2) which penetrate into the submucosa, have a high risk of LN involvement. The invasion of the muscularis mucosa seems to be of paramount importance for the dissemination to the submucosal lymphatic network. There is debate over what constitutes the limit of endoscopic resection. Lesions extending into only the most superficial submucosal layer staged sm1 seem to be critical in this context. A clinical series reported by Manner et al. demonstrated that EMR could be used to treat “low-risk” submucosal sm1 tumors with low-grade tumor differentiation (27). With a mean follow-up of five years, there were no tumor-related deaths. However, two series reported high rate of nodes positive in sm1 tumor: 16.5% for Leers and 21% for Sepsesi (22,28). For tumor invading beyond sm1, existing literature demonstrates that the incidence of LN involvement in patients with T1b cancer ranges between 21% and 50% (22,28-30). For T2 lesion, a review of the outcomes of this subcategory demonstrated that the current approaches to clinical staging resulted in accurate pathologic stage in only 13% of cases. Of the patients inaccurately staged, 63% were overstaged and 37% were understaged. Subsequent recommendations for treatment of cT2N0M0 patients involved proceeding directly to surgery as this would currently be considered a definitive treatment in patients who are accurately staged or overstaged. Patients who are discovered to be understaged can be considered for adjuvant therapy (31).
There is a global agreement for T1b and for T2 cancer to proceed to surgical resection without neoadjuvant therapy that would have a negative effect on survival in this context (32). Indications for esophagectomy in early stage include all incomplete EMR and all failure of endoscopic therapy. Invasion of tumor into the submucosa is still considered a strong indication for esophagectomy, although invasion into the superficial third of the submucosa does not carry the same LN metastasis risk as the deeper two thirds, and potentially could be treated endoscopically (33,34). The risk factors to be considered in the management strategy are listed in Table 1. These risk factors need to be weighed with patient characteristics, patient preferences, available surgical expertise, available endoscopic expertise, and surgical approach options to decide if esophagectomy or endoscopic therapy is appropriate for each case. In this context, a vagal-sparing esophagectomy has been advocated as an alternative to standard resection. Vagal-sparing esophagectomy involves removing the esophagus from the mediastinum with a stripping device that leaves the vagal nerves and the LN in place. In appropriate candidates, vagal-sparing esophageal resection has demonstrated advantages over standard approaches including maintaining meal size, gastric emptying, and BMI (35,36). However, few data are available to promote the technique.
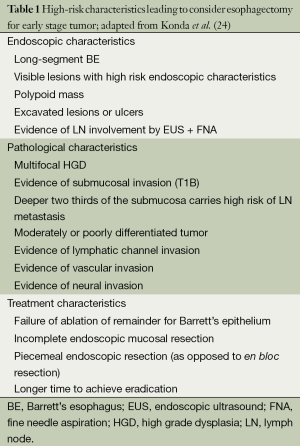
Full table
Indication of neoadjuvant therapy in early stage cancer
Esophagectomy remains the standard treatment of early stage cancer. There are very few data on the benefits of a neoadjuvant treatment for very localized esophageal cancer. The Fédération Francophone de la Cancérologie Digestive (FFCD) 9901 assessed whether preoperative CRT improves outcomes for patients with localized (stages I or II) esophageal cancer (32). From 2000 to 2009, 195 patients were randomized in 30 French centers: 98 were assigned to surgery alone and 97 to neoadjuvant CRT group. Postoperative morbidity rates were 49.5% in surgery group vs. 43.9% in CRT group (P=0.17). The 30 day-mortality rates were 1.1% in surgery group vs. 7.3% in CRT group (P=0.054) respectively. After a median follow-up of 5.7 years, the median survivals were 43.8 in surgery group vs. 31.8 months in CRT group [HR 0.92; 95% confidence interval (CI), 0.63-1.34; P=0.66]. The conclusion of this trial was that neoadjuvant CRT with cisplatin and fluorouracil does not improve overall survival but enhances postoperative mortality rate for patients with stage I or II esophageal cancer compared with surgery alone.
Locally advanced esophageal cancer
Resectable locally advanced esophageal cancer refers to T3-T4A or documented LN involvement (N+ disease) according to the 7th edition of the AJCC (20). At the moment of the diagnosis, vast majority of esophageal tumor are found to be locally advanced cancer. Traditionally, locally advanced esophageal SCC and adenocarcinoma have been managed with surgical resection. Indeed, esophagectomy with radical lymphadenectomy seems to be the best treatment in terms of achieving local control. However, survival was poor, and metastatic disease or locoregional recurrence developed in many patients after surgery. Poor outcomes after surgery alone and analyses of disease recurrence patterns have prompted the addition of adjuvant treatment. However, because esophagectomy is a major procedure with a high rate of postoperative morbidity, multimodal strategy has shifted to neoadjuvant treatment. In some cases and especially for SCC, most oncologist advocate a definitive CRT as first line treatment and reserve surgery as a second therapeutic option in case of failure of the definitive CRT. In this case, the surgery consists in a “salvage esophagectomy”.
Neoadjuvant chemotherapy or CRT
Radiotherapy and chemotherapy could improve survival and disease-free survival before surgery. These improvements can be seen with several aspects. Both treatments are known to improve the control of local or general disease by downstaging cancer and increasing the surgical resecability. Chemotherapy has the potential to eradicate micromestatstic disease by decreasing cancer-cell dissemination. Numerous meta-analyses have been performed to increase the accuracy of comparisons and better estimate potential benefits of neoadjuvant treatment.
Gebski et al. have reported a meta-analysis that evaluated pooled data from clinical trials of neoadjuvant chemotherapy and CRT including both adenocarcinoma and SCC (4). This analysis combined the results of 10 randomized trials of neoadjuvant CRT vs. surgery alone and 8 randomized trials of neoadjuvant chemotherapy vs. surgery alone in patients with locally resectable esophageal carcinoma. The hazard ratio (HR) for all-cause mortality for neoadjuvant chemotherapy was 0.90 (95% CI, 0.81-1.00; P=0.05), indicating a 2-year absolute survival benefit of 7%. For patients with SCC, neoadjuvant chemotherapy did not have a survival benefit [HR for mortality 0.88 (0.75-1.03); P=0.12]. For the adenocarcinoma group, the survival benefit was significant [HR for mortality 0.78 (0.64-0.95); P=0.014]. The HR for all-cause mortality with neoadjuvant CRT vs. surgery alone was 0.81 (95% CI, 0.70-0.93; P=0.002), corresponding to a 13% absolute difference in survival at two years. Analysis of the neoadjuvant CRT studies that had histology data available found a significant benefit over surgery for both histological tumour types: 0.84 (0.71-0.99; P=0.04) for SCC and 0.75 (0.59-0.95; P=0.02) for adenocarcinoma.
In 2011, Sjoquist et al. have published the latest updated meta-analysis (5). The inter-group analysis clearly demonstrated strong arguments for CRT compared to CT in patients with SCC or adenocarcinoma. The updated analysis contained 4,188 patients whereas the previous publication included 2,933 patients. They included all 17 trials from the previous meta-analysis and seven further studies. This updated meta-analysis contains about 3,500 events compared with about 2,230 in the previous meta-analysis (estimated 57% increase). The HR for all-cause mortality for neoadjuvant chemotherapy was 0.87 (0.79-0·96; P=0.005); the HR for SCC only was 0.92 (0.81-1.04; P=0.18) and for adenocarcinoma only was 0. 83 (0. 71-0.95; P=0.01). The HR for all-cause mortality for neoadjuvant CRT was 0.78 (95% CI, 0.70-0.88; P<0.0001); the HR for SCC only was 0.80 (0.68-0.93; P=0.004) and for adenocarcinoma only was 0.75 (0.59-0.95; P=0.02). The HR for the overall indirect comparison of all-cause mortality for neoadjuvant CRT versus neoadjuvant chemotherapy was 0.88 (0.76-1.01; P=0.07).
The Sjoquist’s meta-analysis did not include the latest published phase III trial. The “CROSS trial” compared the outcome of concurrent CRT (carboplatine,plaxitaxel and 41 Gy) followed by surgery and surgery alone (37). A pathological complete response was achieved in 47 of 161 patients (29%) who underwent resection after CRT. Postoperative complications were similar in the two treatment groups, and in-hospital mortality was 4% in both. Median overall survival was 49.4 months in the CRT surgery group versus 24 months in the surgery group. Overall survival was significantly better in the CRT group [HR 0.657 (0.495-0.871; P=0.003)].
To summarize, the optimum neoadjuvant treatment regimen has not been established, because including western and eastern populations, trials used different drugs, doses, and schedules of chemotherapy and radiotherapy. However, there are strong arguments and a global agreement for patients with locally advanced esophageal cancer, that neoadjuvant CRT remains strongly recommended compared to neoadjuvant chemotherapy alone.
CRT: sequential or concomitant?
From the Gebski’s meta-analysis, there was no survival benefit of sequential CRT for patients with SCC [HR for mortality 0.9 (0.72-1.03); P=0.18] (4). The results of sequential CRT were similar to that for patients with SCC assigned neoadjuvant chemotherapy. Concomitant CRT in patients with SCC had a significant benefit [HR for mortality 0.76 (0.59-0.98); P=0.04]. On this basis, the use of concomitant neoadjuvant CRT is strongly recommended compared to sequential CRT.
Neoadjuvant or adjuvant treatment?
The Japan Clinical Oncology Group has conducted randomized, two controlled trials to assess potential benefits of adding adjuvant therapy to surgery in patients with SCC: the JCOG 9204 and the JCOG 9907 (38,39). The JCOG 9204 study assessed the benefit of postoperative adjuvant CT with cisplatin plus 5-FU compared with surgery alone in patients with resectable stage I or II esophageal cancer (38). Overall survival did not differ significantly between the groups (5-year survival rate 52% vs. 61%; P=0.13). Disease-free survival was improved significantly in the patients who received postoperative CT and especially in N+ patients. In the JCOG 9907 study, neoadjuvant CT with cisplatin and 5-FU was compared with postoperative CT with cisplatin and 5-FU in patients with clinical stage II or III esophageal cancer (39). Neoadjuvant CT was found to be superior to postoperative CT in overall survival. The 5-year survival rate was 60% in neoadjvant group vs. 38% in adjuvant group (P=0.013). On the basis of these results, neoadjuvant chemotherapy followed by radical surgery compared to adjuvant strategy is recommended in case of locally advanced SCC.
Neoadjuvant CRT followed by surgery or definitive CRT?
The concept of a definitive CRT was introduced with the results of the Radiation Therapy Oncology Group (RTOG) 8,501 study (40). This trial compared the effect of RT alone (64 Gy) to a scheme of a concurrent CRT (cisplatin, 5-FU, and radiotherapy 50 Gy). The study included both SCC or adenocarcinoma of the esophagus. This study demonstrated the strong sensitivity of SCC to a concomitant CRT. Concomitant CRT resulted in better overall survival and decrease in local failure than RT alone. These results lead a Japanese phase II to assess the effectiveness of definitive CRT (cisplatin, 5-FU, and classic portal radiation 60 Gy) (41). A complete response (CR) was obtained in 68% with a 3-year survival rate of 46%. These results were not superior to those obtained with conventional surgical resection with or without chemotherapy. Two large randomized trials were conducted to compare definitive CRT with neoadjuvant CRT in esophageal SCC (42,43). In a study performed by the German Esophageal Cancer Study Group, the 2-year overall survival results were similar in the surgery (39.9%) and nonsurgery (35.4%) treatment groups (42). A disadvantage of neoadjuvant therapy group was early postoperative mortality, while the definitive CRT in the nonsurgery group was associated with more local relapses. These results were confirmed in another large randomized study performed by FFCD 9102 study where surgery was proposed in responders to CRT. Once again, surgery improved local control, but did not improve survival, because neoadjuvant therapy was associated with increased early mortality (43). An FFCD trial comparing systematic surgery vs. salvage esophagectomy in responders after a neoadjuvant CRT is ongoing in France and it will provide an answer to this important issue.
On the basis on these results, definitive CRT or neoadjuvant CRT followed by surgery seem to have similar long-term results. Despite flaws in these studies, surgery seems to provide a better local control of the tumor but without benefit on long-term outcome. Moreover, this benefit is possible at the cost of major surgery and to the subsequent postoperative mortality.
The particularity of locally advanced signet ring cell adenocarcinoma
Nowadays, in Western countries and for unclear reasons, we assist to a dramatic increase in the incidence of the diffuse form of esophagogastric adenocarcinoma, particularly signet ring cell (SRC) tumors (44,45). Because of their infiltrating and aggressive characteristics, SRC tumors are often diagnosed at a locally advanced stage, with high propensity for peritoneal metastasis and LN invasion (46,47). The problem related to this specific histological subtype remains its innate chemoresistance suggested in gastric cancer (46). In 2010, the FREGAT (French Eso-Gastric Tumors) Working Group carried out a retrospective multicenter study in France of all consecutive esophagogastric cancer treated in 21 centers between 1997 and 2010. Reporting on more than 1,000 patients, survival was significantly shorter in the perioperative chemotherapy group compared to surgery alone, a variable identified as an independent predictor of poor survival and providing evidence of a potential chemoresistance for SRC (47). An alternative option has been suggested with the use of a neoadjuvant CRT (48). This beneficial advantage of CRT was also found in the Sjoquist’s meta-analysis suggesting survival benefit when compared with surgery alone (5). Recently, the FREGAT working group demonstrated in a retrospective study comparing neoadjuvant CRT versus surgery alone in stage III advanced SRC, the benefits of such strategy. There was evidence of significant tumoral, nodal and ypTNM downstaging after neoadjuvant CRT (49). In the neoadjuvant group and in the surgery group, 3-year overall survival was respectively 51% and 21% (P=0.002). The disease recurrence rate was 30.4% in the CRT group compared to 59.5% in surgery group (P=0.015) respectively. In multivariate analysis the sole independent favorable prognostic factor identified was the administration of neoadjuvant CRT (OR: 0.41, P=0.020). Furthers trials evaluating neoadjuvant therapeutic strategies for esophagogastric tumors need to include stratification on SRC histology to prospectively confirm the best treatment strategy.
Salvage esophagectomy
In Japan and in Western countries, medical and radiation oncologists have reported satisfactory results with definitive CRT thus blurring the boundaries of traditional treatment strategies. Burned by unsatisfying related-treatment mortality of surgery, definitive CRT is now considered as treatment option in potentially resectable patients. This has been also motivated by the 15-30% of complete response in the resected specimen after neoadjuvant therapy (5). However, local failure after definitive CRT remains problematic. Persistent or recurrent local disease after definitive CRT remains the greatest drawback of this strategy: 11-26% of patients do not exhibit any morphologic tumor response leading to a dismal prognosis with a median survival at nine months (50). For a subset of carefully selected patients, salvage esophagectomy remains the only curative option.
Locoregional recurrence is defined as tumor detected more than three months after CRT. Persistence is defined by tumor detected within three months in the same site (51). Unfortunately, locoregional control is often quite poor with definitive CRT, and 40% to 60% of the patients have persistent or relapsed tumor at the primary site within one year (43). In this way, salvage esophagectomy stands out as the logical answer for selected patient who received up to 50 Gy of radiation and who are physiologically fit for salvage operation. They can be offered a salvage surgery, a procedure intended to rescue them from an isolated local failure after definitive CRT. Local problems can be related to a neoplastic disease but can also be due to a local toxicity or a mechanical complication.
Previous studies have demonstrated the feasibility of the salvage esophagectomy (50-59). These data suggested that despite the increased morbidity and mortality, a subset of patients were cured after salvage esophagectomy with an acceptable long-term outcome. The decision to proceed with salvage esophagectomy is problematic and each individual case must be considered. Because of the fibrosis due to the high dose of radiotherapy, histological confirmation of the malignancy is difficult to obtain, in less 60% of cases (59). Because the high postoperative mortality, selection of these patients and indications of this salvage procedure must be considered after careful consideration. Initial studies examining the utilization of ‘salvage esophagectomy’ indicated that these procedures were associated with significant increases in operative mortality, respiratory and anastomotic complications and increased length of ICU and in-hospital stay (50-60). A recent pooled-analysis of more than eight studies comprising 954 patients revealed that salvage esophagectomy resulted in significant higher mortality and morbidity rate (Table 2). Salvage resection was associated with a significantly increased incidence of post-operative mortality, anastomotic leak, pulmonary complications and an increased length of hospital stay (60). Much of this concern originated from a historical impression that surgical resection outside of 4-8 weeks following radiotherapy or CRT was more technically challenging and associated with increased postoperative morbidity and mortality. This opinion has recently been challenged (61) and there are now several publications demonstrating that selected utilization of salvage surgery in patients who have failed definitive CRT for SCC can be done with acceptable levels of both mortality and morbidity (51,54,59). Special attention has to be paid of the volume dose of radiation. Salvage surgery is a highly invasive and morbid operation after a volume dose of radiation exceeding 55 Gy (59). It should be noted, however, that a randomized clinical trial that assessed long-term outcomes indicated that definitive radiation chemotherapy had the potential for producing progressive deterioration in pulmonary function when compared to surgery alone (61).

Full table
Minimally invasive esophagectomy (MIE)
Over the last decades, MIE has expanded worldwide. It is estimated that between 15-30% of all esophagectomies use nowadays such procedures (62,63). MIE includes a huge mix of several techniques including hybrid techniques, full MIE and robotic surgery (64). There are now centers who are publishing consecutive series of over 1,000 minimally invasive procedures (65). The most appropriate approach to the esophagectomy will vary from center to center, and the decision should be based on adapting the surgical approach to individual physiologic and tumor-related issues in each patient and referring to centers who have achieved and documented acceptable baseline outcomes (66). It seems likely that importance of MIE will exceed hybrid techniques that have been probably at the onset of the training and the development of the techniques.
There are currently one prospective controlled trial and numerous uncontrolled retrospective comparisons of open versus minimally invasive operations (67-75). All demonstrate the beneficial advantages of the minimally invasive procedures requiring more operative time but being associated with less blood loss and potentially less respiratory complications with a reduced hospital stay. In the TIME trial (67) (Table 3), 56 patients were assigned to open esophagectomy and 59 to a MIE. Sixteen (29%) patients in the open esophagectomy group had pulmonary infection in the first two weeks compared with five (9%) in the minimally invasive group. Nineteen (34%) patients in the open esophagectomy group had pulmonary infection in-hospital compared with seven (12%) in the minimally invasive group. For in-hospital mortality, one patient in the open esophagectomy group died from anastomotic leakage and two in the minimally invasive group from aspiration and mediastinitis after anastomotic leakage. Although operating time was significantly longer in the MIE group than in the open esophagectomy group, blood loss was lower for patients undergoing the minimally invasive procedure. Hospital stay in the MIE group was significantly shorter than that in the open group. These data had been confirmed by others meta-analyses of case control studies performed to date (68-72).

Full table
If the feasibility of MIE seems to be confirmed, the main drawback of the current knowledge in this context remains that MIE have been poorly investigated in term of standardized oncologic criteria’s such as survival, disease-free survival or number of LN retrieved. A recent extensive review of evidence-based surgical treatment of esophageal cancer has highlighted the potential advantages of MIE, but also cautioned that there may be a ‘patient selection bias’ in that patients with less comorbidities and earlier tumors may be more prevalent in reports of the early experience of MIE. In addition, there could also be a ‘publication bias’ in that the published results of minimally invasive surgery will be from the most experienced and successful centers, while other centers who have attempted the transition to minimally invasive techniques with poorer outcomes are less likely to publish (72). In contrast, in the same period that has seen the widespread of MIE, there have been significant improvements in anesthesia, in perioperative management and in standardized esophageal clinical pathways, resulting in a more difficult interpretation of these potential benefits of the MIE (73).
The best information available on oncologic outcome after MIE comes from a meta-analysis from Dantoc et al. (69,70). This review focused on the oncologic merits of MIE techniques compared with conventional open techniques. In the analysis of the data procured from 16 separate case control studies, the capability of surgeons to perform an adequate lymphadenectomy using MIE was established. Evidence points to the capacity of MIE techniques for greater LN yield owing to better visualization of the operative field. In addition, the authors found no statistically significant difference in survival rates between open procedures and MIE. In comparing East vs. West, Western centers had a statistically better LN yield with open vs. MIE, but the difference was not significant in Eastern centers. On survival, Eastern and Western centers showed no statistically significant survival advantage for MIE. Finally, although a lack of standardized and controlled data limits the methods used in this study, the evidence suggests that the use of MIE was no better or worse in achieving similar oncologic outcomes than were open techniques. Further randomized controlled studies are needed to provide credible clinical evidence of the oncologic outcomes of open techniques vs. MIE. Thus, two others phase III trials are currently recruiting and are ongoing: the French’ MIRO trial (74) and the Netherlands’ ROBOT trial (75).
Conclusions
Management of esophageal cancer has been refined since the last decades. What is clear, is surgery continue to play a pivotal role in the treatment of the disease, alone or in combination of multimodal approach. Progress in anesthesia and in surgery has lead to significant decrease of the mortality rate. These improvements in mortality can be seen on national levels in either Western or Eastern countries. Mortality rate of 5% and even under 2% in some experienced centers are increasingly being seen and expected. The progress made in surgery lead surgeons to consider minimal techniques to reduce morbidity and mortality of such high-risk procedures. New techniques of MIE and robotic surgery in a near future will provide opportunity to push the boundary of the indications in very selected group of patients. Based on an commitment of respect the oncological principles including at least a two-stage LN dissection and a specific surgical planning targeted to achieve an R0 resection, these minimal techniques have to provide satisfactory results in term of early and long-term outcome without jeopardizing the disease-free survival. MIE will exceed hybrid techniques and will be compared to robotic esophagectomy. A high-degree of qualification with a high-level of expertise in a high-volume centers seem to be crucial in this context.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. [PubMed]
- Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am 2009;18:469-85. [PubMed]
- Hayeck TJ, Kong CY, Spechler SJ, et al. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus 2010;23:451-7. [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [PubMed]
- Low DE. Evolution in surgical management of esophageal cancer. Dig Dis 2013;31:21-9. [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable esophagealcancer. J Gastrointest Surg 2007;11:1384-93. [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [PubMed]
- Weber WA, Ott K. Imaging of esophageal and gastric cancer. Semin Oncol 2004;31:530-41. [PubMed]
- Rebollo Aguirre AC, Ramos-Font C, Villegas Portero R, et al. 18F-fluorodeoxiglucose positron emission tomography for the evaluation of neoadjuvant therapy response in esophageal cancer: systematic review of the literature. Ann Surg 2009;250:247-54. [PubMed]
- Piessen G, Petyt G, Duhamel A, et al. Ineffectiveness of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of tumor response after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg 2013;258:66-76. [PubMed]
- Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol 2010;8:1037-41. [PubMed]
- Smith BR, Chang KJ, Lee JG, et al. Staging accuracy of endoscopic ultrasound based on pathologic analysis after minimally invasive esophagectomy. Am Surg 2010;76:1228-31. [PubMed]
- Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 2008;14:1479-90. [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [PubMed]
- Das A, Singh V, Fleischer DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340-5. [PubMed]
- Higuchi K, Koizumi W, Tanabe S, et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res 2009;3:153-61. [PubMed]
- Rice TW, Rusch VW, Ishwaran H, et al. Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010 15;116:3763-73.
- Shimada H, Nabeya Y, Matsubara H, et al. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg 2006;191:250-4. [PubMed]
- Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8. [PubMed]
- van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011;60:765-73. [PubMed]
- Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159-64. [PubMed]
- Chennat J, Waxman I. Endoscopic treatment of Barrett’s esophagus: From metaplasia to intramucosal carcinoma. World J Gastroenterol 2010;16:3780-5. [PubMed]
- Semlitsch T, Jeitler K, Schoefl R, et al. A systematic review of the evidence for radiofrequency ablation for Barrett’s esophagus. Surg Endosc 2010;24:2935-43. [PubMed]
- Manner H, May A, Pech O, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol 2008;103:2589-97. [PubMed]
- Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010;210:418-27. [PubMed]
- Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278-88. [PubMed]
- Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg 2009;87:1048-54. [PubMed]
- Rice TW, Mason DP, Murthy SC, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg 2007;133:317-24. [PubMed]
- Mariette C, Seitz JF, Maillard E, et al. Surgery alone versus chemoradiotherapy followed by surgery for localized esophageal cancer: Analysis of a randomized controlled phase III trial FFCD 9901. J Clin Oncol 2010;28:abstr 4005.
- Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570-8. [PubMed]
- Peters FP, Brakenhoff KP, Curvers WL, et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest Endosc 2008;67:604-9. [PubMed]
- Peyre CG, DeMeester SR, Rizzetto C, et al. Vagal-sparing esophagectomy: the ideal operation for intramucosal adenocarcinoma and barrett with high-grade dysplasia. Ann Surg 2007;246:665-71. [PubMed]
- DeMeester SR. New options for the therapy of Barrett’s high-grade dysplasia and intramucosal adenocarcinoma: endoscopic mucosal resection and ablation versus vagal-sparing esophagectomy. Ann Thorac Surg 2008;85:S747-50. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG 9204. J Clin Oncol 2003;21:4592-6. [PubMed]
- Igaki H, Kato H, Ando N, et al. A randomized trial of postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus neoadjuvant chemotherapy for clinical stage II/III squamous cell carcinoma of the thoracic esophagus (JCOG 9907). J Clin Oncol 2008;26:abstr 4510.
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [PubMed]
- Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011;81:684-90. [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [PubMed]
- Kampschöer GH, Nakajima T, van de Velde CJ. Changing patterns in gastric adenocarcinoma. Br J Surg 1989;76:914-6. [PubMed]
- Chirieac LR, Swisher SG, Correa AM, et al. Signet-ring cell or mucinous histology after preoperative chemoradiation and survival in patients with esophageal or esophagogastric junction adenocarcinoma. Clin Cancer Res 2005;11:2229-36. [PubMed]
- Piessen G, Messager M, Leteurtre E, et al. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 2009;250:878-87. [PubMed]
- Messager M, Lefevre JH, Pichot-Delahaye V, et al. FREGAT working group - FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 2011;254:684-93. [PubMed]
- Mariette C, Piessen G, Briez N, et al. Oesophagogastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol 2011;12:296-305. [PubMed]
- Bekkar S, Gronnier C, Messager M, et al. French Eso-Gastric Tumors Working Group-Fédération de Recherche en Chirurgie. The impact of preoperative radiochemotherapy on survival in advanced esophagogastric junction signet ring cell adenocarcinoma. Ann Thorac Surg 2014;97:303-10. [PubMed]
- Swisher SG, Wynn P, Putnam JB, et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg 2002;123:175-83. [PubMed]
- Nakamura T, Hayashi K, Ota M, et al. Salvage esophagectomy after definitive chemotherapy and radiotherapy for advanced esophageal cancer. Am J Surg 2004;188:261-6. [PubMed]
- Smithers BM, Cullinan M, Thomas JM, et al. Outcomes from salvage esophagectomy post definitive chemoradiotherapy compared with resection following preoperative neoadjuvant chemoradiotherapy. Dis Esophagus 2007;20:471-7. [PubMed]
- Kim JY, Correa AM, Vaporciyan AA, et al. Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg 2012;93:207-12. [PubMed]
- Yoo C, Park JH, Yoon DH, et al. Salvage esophagectomy for locoregional failure after chemoradiotherapy in patients with advanced esophageal cancer. Ann Thorac Surg 2012;94:1862-8. [PubMed]
- Meunier B, Raoul J, Le Prise E, et al. Salvage esophagectomy after unsuccessful curative chemoradiotherapy for squamous cell cancer of the esophagus. Dig Surg 1998;15:224-6. [PubMed]
- Marks JL, Hofstetter W, Correa AM, et al. Salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma. Ann Thorac Surg 2012;94:1126-32. [PubMed]
- Tomimaru Y, Yano M, Takachi K, et al. Factors affecting the prognosis of patients with esophageal cancer undergoing salvage surgery after definitive chemoradiotherapy. J Surg Oncol 2006;93:422-8. [PubMed]
- Oki E, Morita M, Kakeji Y, et al. Salvage esophagectomy after definitive chemoradiotherapy for esophageal cancer. Dis Esophagus 2007;20:301-4. [PubMed]
- D’Journo XB, Michelet P, Dahan L, et al. Indications and outcome of salvage surgery for oesophageal cancer. Eur J Cardiothorac Surg 2008;33:1117-23. [PubMed]
- Markar SR, Karthikesalingam A, Penna M, et al. Assessment of short-term clinical outcomes following salvage esophagectomy for the treatment of esophageal malignancy: systematic review and pooled analysis. Ann Surg Oncol 2014;21:922-31. [PubMed]
- Teoh AY, Yan Chiu PW, Wong TC, et al. Functional performance and quality of life in patients with squamous esophageal carcinoma receiving surgery or chemoradiation: results from a randomized trial. Ann Surg 2011;253:1-5. [PubMed]
- Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus 2009;22:195-202. [PubMed]
- National Oesophago-Gastric Cancer Audit 2010. An Audit of the Care Received by People with Oesophago-Gastric Cancer in England and Wales. Third Annual Report. Available online: http://www.augis.org/pdf/NHS-IC-OGC-Audit-2010-interactive.pdf
- Decker G, Coosemans W, De Leyn P, et al. Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg 2009;35:13-20. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1,000 patients. Ann Surg 2012;256:95-103. [PubMed]
- Javidfar J, Bacchetta M, Yang JA, et al. The use of a tailored surgical technique for minimally invasive esophagectomy. J Thorac Cardiovasc Surg 2012;143:1125-9. [PubMed]
- Biere SS, van Berge Henegouwen MI, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [PubMed]
- Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed]
- Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg 2012;147:768-76. [PubMed]
- Dantoc MM, Cox MR, Eslick GD. Does minimally invasive esophagectomy (MIE) provide for comparable oncologic outcomes to open techniques? A systematic review. J Gastrointest Surg 2012;16:486-94. [PubMed]
- Watanabe M, Baba Y, Nagai Y, et al. Minimally invasive esophagectomy for esophageal cancer: an updated review. Surg Today 2013;43:237-44. [PubMed]
- Lagarde SM, Vrouenraets BC, Stassen LP, et al. Evidence-based surgical treatment of esophageal cancer: overview of highquality studies. Ann Thorac Surg 2010;89:1319-26. [PubMed]
- Low DE, Kunz S, Schembre D, et al. Esophagectomy - it’s not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg 2007;11:1395-402. [PubMed]
- Briez N, Piessen G, Bonnetain F, et al. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial - the MIRO trial. BMC Cancer 2011;11:310. [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [PubMed]


