Combining targeted agents and hypo- and hyper-fractionated radiotherapy in NSCLC
Introduction
Arguably one of the most important objectives for cancer researchers remains the reduction in the millions of years of healthy life lost to lung cancer worldwide each year [estimated at 24.5 million in 2008 (1)] with little impact made on the poor relative survival in recent years (2) and improvements in survival trailing behind other cancers (3). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers. Approximately one third of these patients have early stage disease (stages I and II) at the time of presentation and are usually treated surgically, with radiotherapy being reserved for those who are medically inoperable. Another one third of patients present with advanced disease and radiotherapy is reserved for palliation of symptoms. The remainder of patients present with locally advanced disease (stage III) with the majority being unresectable and the mainstay of treatment is radical intent radiotherapy.
In good performance status patients, the addition of sequentially or concomitant platinum-based chemotherapy is considered as the standard of care in patients with locally advanced disease due to the associated improved outcome (4,5). Importantly, a meta-analysis of over 1,200 patients from six trials comparing concomitant to sequential chemo-radiotherapy reveals the concomitant approach is associated with lower loco-regional disease progression (absolute decrease of 6.1% at five years, from 35.0% to 28.9%) but similar distant disease progression (40.6% and 39.5%, respectively) compared to sequential (6). This suggests an important temporal relationship between the two treatment modalities. The consequent 4.5% increase in 5-year overall survival from 10.6% with sequential to 15.1% with concomitant chemotherapy highlights the opportunity for radio-sensitisation with systemic agents and the relevance of improved local disease control on long term outcome.
However, an estimated 60% of patients with locally advanced disease are not fit enough for concomitant chemo-radiotherapy due to poor performance status and co-morbidities (7). In addition to the less toxic alternative of sequential chemo-radiotherapy, radiotherapy dose escalation has been explored, given conventional doses achieve sub-optimal rates of local disease control with estimates of pathologically persistent tumour following treatment in 60% of patients (8). Tumour control probability modelling suggests that using conventional fractionation (1.8 to 2 Gy daily), a dose of 84 Gy is required to achieve 50% probability of tumour control at three years (9), some 18-24 Gy higher than the standard dose radiotherapy. Unfortunately, preliminary clinical data from the RTOG 0617 randomised phase III trial of conventionally fractionated radiotherapy (with concurrent and consolidation platinum-based chemotherapy +/– cetuximab) comparing standard dose (60 Gy) to high dose (74 Gy) has revealed the conventionally fractionated high dose arm is associated with a higher rate of local disease progression (34% compared to 25%) and shorter median survival (19.5 months compared to 28.7 months) compared to standard dose (10). It is as yet unclear the reason for the detrimental effect of the higher dose arm, but the extended duration of treatment by dose escalating using conventionally fractionated may be an important factor.
The alternative strategy is to intensify radiotherapy dose using modified fractionation schedules and reduced overall length of the treatment course with the aim of reducing the effect of accelerated tumour cell repopulation during treatment (11,12). The number of fractions given each day can be increased from one to two or three with at least a 6-hour gap in-between (hyper-fractionation) or the number of daily fractions given can be decreased by increasing the dose per fraction (hypo-fractionation). Such schedules increase the biologically effective dose (BED) (13) delivered to the tumour. Experience with extreme hypo-fractionation in stereotactic ablative radiotherapy for early stage disease demonstrates that a BED of over 100 Gy (using a ratio of 10 for tumour linear to quadratic radio-sensitivity) is required to achieve local disease control rates in excess of 90% (14,15). A recent meta-analysis of over 2,000 patients, of which >80% had stage III disease, from eight trials comparing modified to conventional fractionation radiotherapy schedules reveals modified fractionation is associated with improved overall survival at five years (absolute increase of 2.5%, from 8.3% to 10.8%) compared to standard fractionation schedules and importantly, good compliance with the modified regimens (16). Additionally accelerated radiotherapy is associated with higher pathological complete resection rates than conventional fractionation in patients with stage III NSCLC treated with tri-modality therapy (17). The optimal modified fractionation schedule is yet to be clarified, however accelerated schedules to a total dose of 60-66 Gy are considered optimal for patients considered unsuitable for concomitant chemo-radiotherapy (18).
With the recent increase in understanding of the molecular biology of NSCLC and experience of the use of targeted agents in the advanced disease setting, a number of published studies report on combining targeted agents into radical treatment schedules for locally advanced disease, from addition to concomitant chemo-radiotherapy in good performance status patients to combination with radiotherapy alone in elderly or poor performance status patients. Published studies in the various clinical settings are discussed below.
Molecular biology of NSCLC and epidermal growth factor receptor (EGFR) inhibitors
EGFR is one of a family of four structurally similar tyrosine kinase-associated receptors which comprise the human epidermal growth factor receptor (HER) family. EGFR (HER1 or ERBB1) was the first to be described in humans, and identified to be a protein comprising an extracellular ligand-binding domains, trans-membrane domain and an intracellular tyrosine kinase domain (19). Each receptor must homo- or hetero-dimerise to activate the intrinsic kinase activity and phosphorylate tyrosine residues on the C-terminal tail, activating intracellular signalling pathways. Epidermal growth factor expression has long been regarded as a poor prognostic factor in NSCLC, suggesting its potential as a therapeutic target (20,21).
Since then, a number of small molecule reversible and more recently irreversible EGFR tyrosine kinase motif inhibitors (TKIs) have been developed, with gefitinib and erlotinib both demonstrating modest activity in EGFR wild-type advanced NSCLC (22,23), leading to licensing for erlotinib. The discovery of constitutionally activating somatic EGFR mutations mapping to the kinase domain in 2004 (24,25) changed drug development strategies, with gefitinib, erlotinib and afatinib now licensed for EGFR TKI naïve advanced NSCLC, with an overwhelming consistent evidence from eight randomized trials demonstrating their superior efficacy over chemotherapy in advanced NSCLC. In this setting, toxicities of EGFR TKIs are more manageable than chemotherapy, and toxic fatalities rare usually at up to 3%. Moreover, there seems to be no obvious difference in proportion of grade 3-5 toxicities between the three agents. The most significant serious adverse event reported in EGFR-TKI development was initially pneumonitis. However, with greater experience of use of these agents in the advanced disease setting, rates of grade 3-5 pneumonitis are routinely observed at up to 3% of most trial series, with no clear differences between the agents, but a possible geographical distribution, with increased events reported from East Asian series (26). Whether this reflects pharmacogenomic differences or differing clinical diagnostic interpretation remains unresolved.
Unlike the success of the EGFR-TKIs, targeting through antibody inhibition has proven more problematic in advanced NSCLC. Whilst preclinical models demonstrated the activity of anti-EGFR monoclonal antibodies (MAbs) against several carcinoma cell lines, with synergistic activity in combination with cisplatin (27), despite encouraging phase II studies (28) two large randomized phase III trials in advanced NSCLC (29,30) demonstrated little or no survival advantage for the addition of cetuximab to standard platinum-doublet chemotherapy, although subsequent post-hoc analyses suggested potential activity contingent on extent of EGFR expression (31). EGFR MAbs are therefore not standard in advanced NSCLC.
For stage III NSCLC, the combination of EGFR inhibitors and radiotherapy has considerable scientific rationale, despite some of the efficacy concerns identified through advanced disease trials. A positive correlation has been demonstrated between EGFR expression and tumour radio-resistance (32) and the magnitude of over-expression has been correlated with the degree of resistance (33). Radiation damage results in increased EGFR expression and subsequent augmentation of down-stream pathways (34,35). Pre-clinical evidence suggests EGFR blockade potentiates tumour radio-sensitivity. Cetuximab has demonstrated the ability to modulate tumour proliferation, apoptosis and inhibit deoxyribonucleic acid (DNA) repair following irradiation (36-39). Gefitinib has been shown to inhibit the radiation-induced activation of DNA-dependent protein kinase and potentiate radiation response (40,41). Erlotinib similarly causes radio-sensitization potentially through a number of effects including increased apoptosis, cell cycle arrest, and DNA damage repair changes (42). Other mechanisms postulated include micro-environmental changes mediated through decreased vascular endothelial growth factor messenger ribonucleic acid (VEGF mRNA) and protein expression, and blunted hypoxia-inducible factor 1-alpha (HIF-1α) induction (43), with studies of gefitinib (44) and cetuximab (45) demonstrating improved oxygenation.
EGFR inhibitors with conventional fractionation radical radiotherapy alone
In the clinical setting, subsequent to the encouraging improved outcomes with minimal additional toxicity in locally advanced head and neck cancer patients treated with radical radiotherapy combined with cetuximab compared to radiotherapy alone (46), similar studies have been carried out in patients with locally advanced NSCLC. Given the patient population offered radiotherapy alone tend to be elderly and/or with poor performance status, the N0422 phase II single arm study of radical radiotherapy (60 Gy) combined with concomitant cetuximab is interesting (47) (Table 1). The cohort of 57 patients with stage III NSCLC who were considered unfit for combined chemo-radiotherapy included either patients aged 65 years or older with an ECOG performance status of 0-1 or patients of any age with a performance status of 2. Fifty patients (86%) completed the entire treatment and there were no treatment related deaths. Grade 3/4 toxicities were experienced by 31 (54%) patients, with the most common side effects being fatigue (9%) and dyspnoea (9%). The median survival of the cohort was 15.1 (95% CI: 31.1-19.3) months. Of note, patients in this study were not staged with positron emission tomography (PET) scans and outdated radiotherapy techniques were used. A similar smaller single arm phase II study, the Near trial, treated 30 patients with stage III NSCLC, who were considered unfit for or who had refused combined chemo-radiotherapy, with radical radiotherapy (66 Gy) combined with concomitant cetuximab followed by maintenance cetuximab (48) (Table 1). The median age of this cohort was younger at 71 years and all patients had a Karnofsky performance status of ≥70%, however, the median survival was encouraging at 19.6 (95% CI: 11.5-24.7) months. Treatment completion rate and grade 3/4 toxicity rates were similar at 90% (27 patients) and 40% (12 patients), respectively, with the most common side effect being pneumonia (10%). There were however three deaths (myocardial infarction, bacterial endocarditis related sepsis, pulmonary embolus following deep vein thrombosis) reported as unlikely related to the treatment. Both studies included elective nodal irradiation up to 40-50 Gy, however in contrast to the first study, patients in the Near trial were staged with PET scans and modern radiotherapy techniques were used, including intensity modulated radiotherapy (IMRT) and cone beam CT image guided delivery. It is also noted that while the median percentage of normal lung planned to receive 20 Gy (V20) in this cohort of patients was 26%, the range extended up to 60% and therefore included patients at high risk for pulmonary complications due to the radiotherapy (51). Given the skin toxicity rates associated with cetuximab, there is interest in newer EGFR MAbs that demonstrate a lower incidence of skin complications, with phase I studies of nimotuzumab in the palliative radiotherapy setting for NSCLC patients demonstrating feasibility and tolerance (52,53).
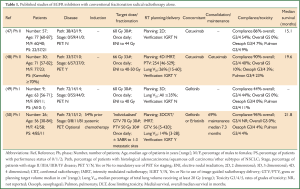
Full table
Studies of erlotinib and gefitinib in combination with radical radiotherapy alone in locally advanced NSCLC have raised concerns about pulmonary toxicity. In particular, a phase II study from Japan (49) (Table 1) on good performance status patients with a median age of 54 years was closed early due to toxicity concerns. Of the nine patients with stage III NSCLC recruited to the study, seven received gefitinib concurrently with thoracic radiotherapy (60 Gy). Three dimensional (3D) conformal planning was used and all plans had a lung V20 ≤35%. Despite this, two of these patients experienced acute pulmonary toxicity (grade 1 and 3) after approximately 30 Gy had been delivered. In contrast, another phase II study from China (50) (Table 1) studied 26 patients with stage III or IV disease, treated with ‘individualised’ radical radiotherapy in combination with either erlotinib or gefitinib. The patients were a heterogeneous group with only 5 (19%) patients having stage III disease. The 21 (81%) patients with stage IV disease had up to three organs treated with stereotactic ablative radiotherapy in addition to radical thoracic radiotherapy given concurrently with the EGFR tyrosine kinase inhibitor. However, treatment was completed as planned in 96% of patients and grade 3/4 pulmonary toxicity rates were acceptable at 4%. The whole cohort had a promising median survival of 21.8 (95% CI: 8.5-35.1) months. Additional toxicity concerns with erlotinib, published in abstract only, come from a small phase I/II Canadian study of erlotinib given concurrently with radical radiotherapy (60 Gy) in poor risk patients with PS 2 or weight loss >5% (54). This study was terminated early due to grade 3-5 pulmonary toxicity in two of five patients.
EGFR inhibitors with conventional fractionation sequential chemo-radiotherapy
An early phase I study demonstrated the safety of combining cetuximab with radical radiotherapy (64 Gy) following induction platinum-based chemotherapy in 12 patients with stage III NSCLC (55) (Table 2). One patient died of bronchopneumonia during treatment and two others experienced grade 3 toxicity (a fatigue and a pneumonitis). All patients radiotherapy plans had a lung V20 <30% (median 22%).
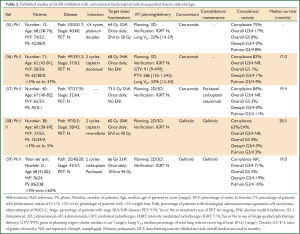
Full table
Subsequently a single arm phase II study, the Satellite trial, treated 71 patients with stage III NSCLC using a combination of cetuximab and radical radiotherapy (68 Gy) following induction chemotherapy (56) (Table 2). The patients were of good performance status [0-1] with a relatively low median age of 62 years, however 37% had significant weight loss prior to treatment, a documented poor prognostic factor (60,61). Interestingly, this study omitted elective nodal irradiation, yet despite this PTV volumes up to 1,543 cm3 (median 586 cm3) were treated and lung V20 parameters up to 54% (median 33%) were documented. Importantly, the study reports high compliance rates, low severe toxicity and a median overall survival of 17 (95% CI: 14.0-23.0) months in the whole cohort and a median survival of 24 months in the patients with <5% weight loss prior to treatment. Impact on health related quality of life with the combination also appears reasonable (62). Of note, the one patient with grade 5 toxicity developed pneumonitis soon after treatment and had a lung V20 of 41%, higher than the recommended QUANTEC constraint of 35% (51). Recently a further phase II study of 40 patients with stage II NSCLC reported on experience of cetuximab with concurrent radiotherapy (73.5 Gy) followed by cetuximab and consolidation chemotherapy with paclitaxel and carboplatin (57) (Table 2). The radiotherapy volumes and normal tissue constraints are not reported however one patient died from pneumonitis after 56 Gy of radiotherapy. Overall median survival was 19.4 (95% CI: 15.4-26) months and interestingly no oesophageal toxicity > grade 2 was observed.
Again concerns over pulmonary toxicity have been raised in studies of EGFR TKIs in combination with radical radiotherapy given sequentially with systemic chemotherapy. A Japanese phase II study, JCOG 0402 trial, in 38 good performance status patients with stage III NSCLC and median age of 60 years received gefitinib concurrently with radical radiotherapy (60 Gy) following two cycles of platinum-based induction chemotherapy (58) (Table 2). Compliance with completing the planned concomitant phase of treatment was low at 63% and a patient (3%) developed grade 3 pneumonitis. However, a promising median survival rate of 28.5 (95% CI: 22.5-38.2) months was reported. The CALEB 30106 phase II study evaluated the addition of gefitinib concurrently with radical sequential or concomitant chemo-radiotherapy to patients with stage III NSCLC, based on initial assessment of prognositic factors (59). Patients considered as ‘poor risk’ in the study were those with a PS of 2 and/or weight loss of ≥5%. These patients were treated similarly to in the Japanese study, with two cycles of platinum-based chemotherapy followed by gefitinib given concurrently with radical radiotherapy (66 Gy). The grade 3/4 pulmonary toxicity rate was 10% with grade 5 pulmonary toxicity rate of 5%. The median survival was 19 (95% CI: 9.9-28.4) months. In both studies PET staging was not mandated and 2D radiotherapy planning was permitted with comparable elective nodal irradiation included to 40-44 Gy. An additional confounding factor for the studies is that in both protocols patients were additionally offered maintenance gefitinib. These studies were designed prior to the reporting of the randomised phase III SWOG S0023 trial of concurrent chemo-radiotherapy and consolidation docetaxel with or without maintenance gefitinib in stage III NSCLC, demonstrating inferior survival for the maintenance gefitinib arm (63).
EGFR inhibitors with conventional fractionation concomitant chemo-radiotherapy
The addition of cetuximab to concomitant chemo-radiotherapy has also been studied in patients with locally advanced NSCLC. The phase II RTOG 0324 study treated 87 good performance status patients radical radiotherapy (63 Gy) and concomitant and consolidation carboplatin, paclitaxel and cetuximab (64) (Table 3). The majority of patients were staged with PET and all had 3D conformal radiotherapy. Compliance with treatment was 68% and grade 3/4 toxicity rates were acceptable, however there were six deaths (7%) considered as related to the treatment and at leastthree of these were pulmonary in nature. The median survival was encouraging at 22.7 (95% CI: 15.3-30.4) months. Another phase II study in 101 good performance status patients with locally advanced NSCLC compared high-dose radical radiotherapy (70 Gy) given with concomitant carboplatin and pemetrexed chemotherapy with or without cetuximab, followed by maintenance pemetrexed. PET staging was mandated and 3D or 4D radiotherapy was used without elective nodal irradiation. Compliance was similarly just over 50% in both arms with acceptable grade 3/4 toxicity rates. There were two (4%) patients with grade 5 toxicities in the arm without cetuximab and three (6%) patients in the cetuximab arm, all pulmonary related. The median survival rates were 21.2 and 25.2 months in the non-cetuximab versus cetuximab arms, respectively. The patients were highly selected which may account in part for the higher than anticipated median survival in the non-cetuximab arm. It is important to note this study was designed before lack of efficacy of pemetrexed in squamous histology was known (70). Also there is concern about the effect of the high-dose of radiotherapy used in this study, given in standard 2 Gy daily fractions, due to the recent preliminary results from the subsequent phase III RTOG 0617 study. The RTOG 0617 trial treated 544 patients with locally advanced NSCLC using radical radiotherapy with concomitant carboplatin and paclitaxel chemotherapy followed by consolidation chemotherapy and randomised patients in a 2×2 factorial design between an escalated dose of 74 Gy compared to 60 Gy in 2 Gy daily fractions and between concomitant cetuximab or not. The initial results of the radiotherapy dose analyses demonstrated a worse prognosis in the high-dose compared to standard-dose radiotherapy arm (10), with an 18-month overall survival of 53.9% versus 66.9%, respectively. Recently, the initial results of the cetuximab analyses were also presented (10) and unfortunately no significant difference was observed in median survival or 18 month overall survival between the cetuximab and non-cetuximab arms (23.1 versus 23.5 months and 60.8% versus 60.2%, respectively). The addition of cetuximab was however associated with increase toxicity compared to the non-cetuximab arm (≥ grade 3 non-haematological 70.5% versus 50.7% and ≥ grade 4 35.8% versus 28.2%, respectively).
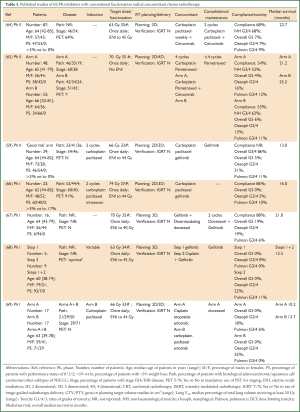
Full table
Phase I studies of erlotinib and gefitinib given with concomitant chemo-radiotherapy for locally advanced disease have demonstrated feasibility of the combination with both standard (68,69) and high-dose (66,67) conventionally fractionated radiotherapy, although the associated medial survivals reported in these studies have been disappointing (~12-16 months) (Table 3). Again confounding factors are noted including for example, lack of PET staging and use of maintenance gefitinib (63) in some studies. In addition, the CALEB 30106 phase II study discussed above in relation to combination of gefitinib given with sequential chemo-radiotherapy, treated the ‘good-risk’ patients, defined as PS 0-1 with <5% weight loss, with two cycles of induction carboplatin and paclitaxel chemotherapy followed by concomitant gefitinib and chemo-radiotherapy to 66 Gy in standard fractionation, followed by maintenance gefitinib. The median overall survival was poor at 13 (95% CI: 8.5-17.2) months and worse than the median survival of 19 (95% CI: 9.9-28.4) months observed in the ‘poor-risk’ patients treated sequentially.
Other targeted agents and radiotherapy for NSCLC
Considerable pre-clinical rationale exists to combine other targeted therapeutics with radiotherapy. The phosphoinositol 3-kinase (PI3K)/Akt/mTOR pathway is transforming for some NSCLC and a number of inhibitors of components of this pathway are in development for advanced NSCLC. Some of these have been shown to be radio-sensitizers in non-NSCLC models (71). Perhaps the best investigated includes abrogation of the tumour microvasculature by vascular disrupting agents (e.g., ZD6126) or anti-angiogenic agents (e.g., bevacizumab). VEGF is known to be upregulated by irradiation and VEGF inhibition is associated with increased tumour control after irradiation in pre-clinical models (72). However, early phase studies have raised toxicity concerns about combinations of agents targeting tumour vasculature or angiogenesis with radiotherapy in NSCLC patients (73) whereas early phase studies of radiotherapy combined with agents targeting tumour cell proliferation and survival pathways demonstrate feasibility (74,75). A recent review highlights the number of pre-clinical and ongoing early phase clinical studies assessing targeting agents in NSCLC patients (76). With the rapidly expanding availability of novel targeted agents and growing experience of these agents in the advanced disease setting, careful consideration of the optimal agents to combine with radiation and study design remains paramount to maximise therapeutic gain and avoid undue toxicity. Guidelines have been published to provide a framework for assessment of novel radio-sensitizers in the pre-clinical and early phase clinical setting (77).
Of the different exploitable mechanisms (78) by which a drug may interact with radiotherapy to improve the therapeutic ratio, it may be that NSCLC patients identified as harbouring an oncogenic driver mutation that confers sensitivity to a specific targeted agent [e.g., echinoderm microtubule-associated protein-like 4 and anaplastic lymphoma kinase gene translocation (EML4-ALK) and ALK TKI crizotinib] will benefit from treatment schedule aimed at maximising spatial co-operation of treatment modalities whereas those without an identifiable mutation may derive benefit from a schedule aimed at maximising the concomitant radio-sensitising approach of combining novel agent with radiotherapy. The central role of DNA damage response to radiotherapy and whether this effect can be modulated by targeted agents remains an important area of research (79). Modulation of the effect of radiation rather than targeting specific driver mutations is also of research interest given the emerging issues of tumour heterogeneity (80).
Targeted agents with altered fractionation radiotherapy in NSCLC
Whilst the majority of studies of targeted agents with radiotherapy in NSCLC have also included concomitant chemotherapy, it is important to maintain a focus on studies of radiotherapy and targeted agent without additional chemotherapy or with sequential chemotherapy for the important group of patients with locally advanced NSCLC who are elderly, have poor performance status or multiple co-morbidities (7). With evidence that modified fractionation schedules are associated with improved outcome compared to conventional fractionation in NSCLC (16) and the experience to date of combining cetuximab with conventionally fractionated radiotherapy alone or sequential chemo-radiotherapy suggesting feasibility with acceptable toxicity, studies of cetuximab with modified fractionation radiotherapy in these settings are warranted. Patient selection remains important with accurate staging and reporting of important prognositic factors in addition to patient demographics to assist the reproducibility of treatment results in the wider population.
Given the initial results from the phase III RTOG 0617 study, there does not appear to be a role for the additional of cetuximab in combination with standard dose concurrent chemo-radiotherapy using conventional fractionation. Interestingly, no significant interaction between the radiotherapy dose and the addition of cetuximab were observed. The question remains as to whether cetuximab can be safely added to modified fractionation schedule chemo-radiotherapy and whether this provides any benefit.
Additional considerations
When considering the total dose of radiation prescribed for a given schedule, it is important to consider that locally advanced NSCLC encompasses a heterogenous population of individuals with differing volume, location and extent of disease. Recently the concept of isotoxic dose escalation was introduced, moving away from a fixed radiotherapy dose prescription for all patients to a tailored prescription based on the surrounding normal tissue dose constraints, predicting a certain acceptable probability of toxicity (81). Use of this approach in modified fractionation radiotherapy with sequential or concomitant chemotherapy demonstrates promising results the in phase II setting (82-84). The study of the addition of targeted agents to isotoxic dose escalated accelerated radiotherapy schedules is an interesting area of ongoing research.
For trial design, patient selection remains important and patients need to be optimally staged and stratified based on prognostic variables to ensure the results are repeatable in the wider patient population. State-of-the-art radiotherapy techniques for planning and delivery, including IMRT and image-guided radiotherapy (IGRT), stand to optimise the therapeutic window. Detailed reporting of radiotherapy planning and delivery parameters will reduce the heterogeneity in studies discussed above and permit optimal comparison between studies and reproducibility of outcomes.
Further work is required to improve understanding of the mechanisms of response and toxicity using targeted agents with radiation and to assess for early predictors of response and toxicity, particularly with respect to fraction-size sensitivity with the increasing use of altered fractionation radiotherapy schedules.
Conclusions
Advances in the molecular understanding of NSCLC have accelerated in recent years and the era of personalised medicine in systemic treatment, particularly in advanced disease, has become a reality. At the same time, advances in technology and imaging have led to improvements in patient selection and in accuracy of radical radiotherapy planning and delivery for locally advanced NSCLC. The combination of individualised biological optimisation using novel targeted agents with physical optimisation using state-of-the-art radical (chemo-) radiotherapy, including accelerated-fractionation schedules and individualised radiotherapy dose-prescriptions, stands to improve outcomes in the heterogeneous population of patients with unresectable locally advanced NSCLC.
Acknowledgements
This review work was supported by The Royal Marsden NHS Foundation Trust which received a proportion of its funding from the NHS Executive; the views expressed in this publication are those of the authors and not necessarily those of the NHS Executive. We acknowledge NHS funding to the NIHR Biomedical Research Centre.
Disclosure: FM reports no actual or potential conflicts of interest. SP has been a non-reimbursed consultant to Astra Zeneca, Boehringer Ingelheim and Roche.
References
- Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 2012;380:1840-50. [PubMed]
- Cancer Research UK; 2013 (updated 2013; cited 2013); Available online: http://info.cancerresearchuk.org/cancerstats/survival
- Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127-38. [PubMed]
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [PubMed]
- Aupérin A, Le Péchoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006;17:473-83. [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [PubMed]
- De Ruysscher D, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol 2009;20:98-102. [PubMed]
- Groen HJ, van der Leest AH, Fokkema E, et al. Continuously infused carboplatin used as radiosensitizer in locally unresectable non-small-cell lung cancer: a multicenter phase III study. Ann Oncol 2004;15:427-32. [PubMed]
- Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999;24:31-7. [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. A randomised phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III NSCLC: Results on radiation dose in RTOG 0617. J Clin Oncol 2013;31:abstr 7501.
- Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 1988;27:131-46. [PubMed]
- Suwinski R, Withers HR. Time factor and treatment strategies in subclinical disease. Int J Radiat Biol 2003;79:495-502. [PubMed]
- Fowler JF. 21 years of biologically effective dose. Br J Radiol 2010;83:554-68. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [PubMed]
- Ohri N, Werner-Wasik M, Grills IS, et al. Modeling local control after hypofractionated stereotactic body radiation therapy for stage I non-small cell lung cancer: a report from the elekta collaborative lung research group. Int J Radiat Oncol Biol Phys 2012;84:e379-84. [PubMed]
- Mauguen A, Le Pechoux C, Saunders MI, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol 2012;30:2788-97. [PubMed]
- Pöttgen C, Eberhardt W, Graupner B, et al. Accelerated hyperfractionated radiotherapy within trimodality therapy concepts for stage IIIA/B non-small cell lung cancer: Markedly higher rate of pathologic complete remissions than with conventional fractionation. Eur J Cancer 2013. [Epub ahead of print]. [PubMed]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi89-98. [PubMed]
- Buhrow SA, Cohen S, Garbers DL, et al. Characterization of the interaction of 5'-p-fluorosulfonylbenzoyl adenosine with the epidermal growth factor receptor/protein kinase in A431 cell membranes. J Biol Chem 1983;258:7824-7. [PubMed]
- Veale D, Ashcroft T, Marsh C, et al. Epidermal growth factor receptors in non-small cell lung cancer. Br J Cancer 1987;55:513-6. [PubMed]
- Veale D, Kerr N, Gibson GJ, et al. The relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long term survival. Br J Cancer 1993;68:162-5. [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Hirsch FR, Jänne PA, Eberhardt WE, et al. Epidermal growth factor receptor inhibition in lung cancer: status 2012. J Thorac Oncol 2013;8:373-84. [PubMed]
- Fan Z, Baselga J, Masui H, et al. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res 1993;53:4637-42. [PubMed]
- Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol 2008;19:362-9. [PubMed]
- Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010;28:911-7. [PubMed]
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [PubMed]
- Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 2012;13:33-42. [PubMed]
- Contessa JN, Reardon DB, Todd D, et al. The inducible expression of dominant-negative epidermal growth factor receptor-CD533 results in radiosensitization of human mammary carcinoma cells. Clin Cancer Res 1999;5:405-11. [PubMed]
- Akimoto T, Hunter NR, Buchmiller L, et al. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res 1999;5:2884-90. [PubMed]
- Schmidt-Ullrich RK, Valerie K, Fogleman PB, et al. Radiation-induced autophosphorylation of epidermal growth factor receptor in human malignant mammary and squamous epithelial cells. Radiat Res 1996;145:81-5. [PubMed]
- Schmidt-Ullrich RK, Valerie KC, Chan W, et al. Altered expression of epidermal growth factor receptor and estrogen receptor in MCF-7 cells after single and repeated radiation exposures. Int J Radiat Oncol Biol Phys 1994;29:813-9. [PubMed]
- Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res 1999;59:1935-40. [PubMed]
- Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res 2000;6:2166-74. [PubMed]
- Milas L, Mason K, Hunter N, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res 2000;6:701-8. [PubMed]
- Raben D, Helfrich B, Chan DC, et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res 2005;11:795-805. [PubMed]
- Li L, Wang H, Yang ES, et al. Erlotinib attenuates homologous recombinational repair of chromosomal breaks in human breast cancer cells. Cancer Res 2008;68:9141-6. [PubMed]
- She Y, Lee F, Chen J, et al. The epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 selectively potentiates radiation response of human tumors in nude mice, with a marked improvement in therapeutic index. Clin Cancer Res 2003;9:3773-8. [PubMed]
- Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005;65:3328-35. [PubMed]
- Pore N, Jiang Z, Gupta A, et al. EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res 2006;66:3197-204. [PubMed]
- Solomon B, Binns D, Roselt P, et al. Modulation of intratumoral hypoxia by the epidermal growth factor receptor inhibitor gefitinib detected using small animal PET imaging. Mol Cancer Ther 2005;4:1417-22. [PubMed]
- Krause M, Ostermann G, Petersen C, et al. Decreased repopulation as well as increased reoxygenation contribute to the improvement in local control after targeting of the EGFR by C225 during fractionated irradiation. Radiother Oncol 2005;76:162-7. [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [PubMed]
- Jatoi A, Schild SE, Foster N, et al. A phase II study of cetuximab and radiation in elderly and/or poor performance status patients with locally advanced non-small-cell lung cancer (N0422). Ann Oncol 2010;21:2040-4. [PubMed]
- Jensen AD, Munter MW, Bischoff HG, et al. Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity-modulated RT radiotherapy and cetuximab: the NEAR trial. Cancer 2011;117:2986-94. [PubMed]
- Okamoto I, Takahashi T, Okamoto H, et al. Single-agent gefitinib with concurrent radiotherapy for locally advanced non-small cell lung cancer harboring mutations of the epidermal growth factor receptor. Lung Cancer 2011;72:199-204. [PubMed]
- Wang J, Xia TY, Wang YJ, et al. Prospective study of epidermal growth factor receptor tyrosine kinase inhibitors concurrent with individualized radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e59-65. [PubMed]
- Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6. [PubMed]
- Bebb G, Smith C, Rorke S, et al. Phase I clinical trial of the anti-EGFR monoclonal antibody nimotuzumab with concurrent external thoracic radiotherapy in Canadian patients diagnosed with stage IIb, III or IV non-small cell lung cancer unsuitable for radical therapy. Cancer Chemother Pharmacol 2011;67:837-45. [PubMed]
- Choi HJ, Sohn JH, Lee CG, et al. A phase I study of nimotuzumab in combination with radiotherapy in stages IIB-IV non-small cell lung cancer unsuitable for radical therapy: Korean results. Lung Cancer 2011;71:55-9. [PubMed]
- Wan J, Cohen J, Agulnik J, et al. Unexpected high lung toxicity from radiation pneumonitis in phase I/II trial of concurrent erlotinib with limited field radiation for intermediate prognosis patients with stage III or inoperable stage IIB NSCLC. Int J Rad Onc Biol Phys 2009;75:abstr 235.
- Hughes S, Liong J, Miah A, et al. A brief report on the safety study of induction chemotherapy followed by synchronous radiotherapy and cetuximab in stage III non-small cell lung cancer (NSCLC): SCRATCH study. J Thorac Oncol 2008;3:648-51. [PubMed]
- Hallqvist A, Wagenius G, Rylander H, et al. Concurrent cetuximab and radiotherapy after docetaxel-cisplatin induction chemotherapy in stage III NSCLC: satellite--a phase II study from the Swedish Lung Cancer Study Group. Lung Cancer 2011;71:166-72. [PubMed]
- Ramalingam SS, Kotsakis A, Tarhini AA, et al. A multicenter phase II study of cetuximab in combination with chest radiotherapy and consolidation chemotherapy in patients with stage III NSCLC. Lung Cancer 2013;81:416-21. [PubMed]
- Niho S, Ohe Y, Ishikura S, et al. Induction chemotherapy followed by gefitinib and concurrent thoracic radiotherapy for unresectable locally advanced adenocarcinoma of the lung: a multicenter feasibility study (JCOG 0402). Ann Oncol 2012;23:2253-8. [PubMed]
- Ready N, Janne PA, Bogart J, et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: cancer and leukemia group B (CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol 2010;5:1382-90. [PubMed]
- Werner-Wasik M, Scott C, Cox JD, et al. Recursive partitioning analysis of 1999 Radiation Therapy Oncology Group (RTOG) patients with locally-advanced non-small-cell lung cancer (LA-NSCLC): identification of five groups with different survival. Int J Radiat Oncol Biol Phys 2000;48:1475-82. [PubMed]
- Vokes EE, Herndon JE 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007;25:1698-704. [PubMed]
- Hallqvist A, Bergman B, Nyman J. Health related quality of life in locally advanced NSCLC treated with high dose radiotherapy and concurrent chemotherapy or cetuximab--pooled results from two prospective clinical trials. Radiother Oncol 2012;104:39-44. [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [PubMed]
- Blumenschein GR Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol 2011;29:2312-8. [PubMed]
- Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol 2011;29:3120-5. [PubMed]
- Stinchcombe TE, Morris DE, Lee CB, et al. Induction chemotherapy with carboplatin, irinotecan, and paclitaxel followed by high dose three-dimension conformal thoracic radiotherapy (74 Gy) with concurrent carboplatin, paclitaxel, and gefitinib in unresectable stage IIIA and stage IIIB non-small cell lung cancer. J Thorac Oncol 2008;3:250-7. [PubMed]
- Center B, Petty WJ, Ayala D, et al. A phase I study of gefitinib with concurrent dose-escalated weekly docetaxel and conformal three-dimensional thoracic radiation followed by consolidative docetaxel and maintenance gefitinib for patients with stage III non-small cell lung cancer. J Thorac Oncol 2010;5:69-74. [PubMed]
- Rothschild S, Bucher SE, Bernier J, et al. Gefitinib in combination with irradiation with or without cisplatin in patients with inoperable stage III non-small cell lung cancer: a phase I trial. Int J Radiat Oncol Biol Phys 2011;80:126-32. [PubMed]
- Choong NW, Mauer AM, Haraf DJ, et al. Phase I trial of erlotinib-based multimodality therapy for inoperable stage III non-small cell lung cancer. J Thorac Oncol 2008;3:1003-11. [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [PubMed]
- Karar J, Maity A. Modulating the tumour microenvironment to increase radiation responsiveness. Cancer Biol Ther 2009;8:1994-2001. [PubMed]
- Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the anti-tumour effects of ionising radiation. Cancer Res 1999;59:3374-8. [PubMed]
- Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010;28:43-8. [PubMed]
- Sarkaria JN, Schwingler P, Schild SE, et al. Phase I trial of sirolimus combined with radiation and cisplatin in non-small cell lung cancer. J Thorac Oncol 2007;2:751-7. [PubMed]
- Schild S, Molina J, Dy G. A Phase I study of bortezomib, paclitaxel, carboplatin (CBDCA) and radiotherapy (RT) for locally advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2010;28:abstr 7085.
- Koh PK, Faivre-Finn C, Blackhall FH, et al. Targeted agents in non-small cell lung cancer (NSCLC): clinical developments and rationale for the combination with thoracic radiotherapy. Cancer Treat Rev 2012;38:626-40. [PubMed]
- Harrington KJ, Billingham LJ, Brunner TB, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br J Cancer 2011;105:628-39. [PubMed]
- Bentzen SM, Harari PM, Bernier J. Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat Clin Pract Oncol 2007;4:172-80. [PubMed]
- Powell C, Mikropoulos C, Kaye SB, et al. Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitisers. Cancer Treat Rev 2010;36:566-75. [PubMed]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-92. [PubMed]
- van Baardwijk A, Bosmans G, Boersma L, et al. Individualized radical radiotherapy of non-small-cell lung cancer based on normal tissue dose constraints: a feasibility study. Int J Radiat Oncol Biol Phys 2008;71:1394-401. [PubMed]
- De Ruysscher D, van Baardwijk A, Steevens J, et al. Individualised isotoxic accelerated radiotherapy and chemotherapy are associated with improved long-term survival of patients with stage III NSCLC: a prospective population-based study. Radiother Oncol 2012;102:228-33. [PubMed]
- van Baardwijk A, Reymen B, Wanders S, et al. Mature results of a phase II trial on individualised accelerated radiotherapy based on normal tissue constraints in concurrent chemo-radiation for stage III non-small cell lung cancer. Eur J Cancer 2012;48:2339-46. [PubMed]
- van Baardwijk A, Wanders S, Boersma L, et al. Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol 2010;28:1380-6. [PubMed]


