Accelerated dose escalation with proton beam therapy for non-small cell lung cancer
Introduction
Local tumor control remains a substantial challenge in many cases of non-small cell lung cancer (NSCLC). For patients with early-stage disease, the advent of stereotactic ablative body radiation (SABR) for definitive therapy has drastically reduced the rate of locoregional recurrence (1), but some tumors, particularly those that are large or centrally located, remain challenging to treat because of the risk of severe toxicity (2). For patients with locally advanced disease, concurrent chemotherapy and radiation have been shown to maximize control and survival outcomes, but many patients are not candidates for this approach because of age, the presence of comorbid conditions, or poor performance status (3,4), and for such patients sequential chemoradiation regimens or radiation given alone at conventionally fractionated doses produces suboptimal results.
Thus, more effective and safe radiation therapy regimens are needed for subsets of patients with early-stage or locally advanced NSCLC. An approach that has been increasingly explored over the past decade has been the use of hypofractionated proton beam therapy (PBT). The energy distribution of protons [as opposed to photon (X-ray- or gamma-ray-) based irradiation] has theoretical advantages over that of photons because of the Bragg peak characteristic of proton particles, which can be exploited to reduce exposure of normal tissues to radiation, particularly at low doses. Under this premise, emerging dosimetric and clinical studies are being undertaken to assess the role of PBT, including hypofractionated regimens as appropriate, for carefully chosen patients.
This review summarizes current evidence regarding the use of hypofractionated PBT for early-stage NSCLC, including use of PBT as an alternative to SABR for patients with T1-T2 node-negative tumors, followed by a discussion of PBT for locally advanced disease, including tumors that involve the mediastinum, and the possibility of using hypofractionated regimens for patients who are not candidates for concurrent chemotherapy. We have endeavored to convey a level-of-evidence-based approach to applying these concepts for specific cases and to outline future paths for research to better determine which patients would derive the greatest benefit from hypofractionated PBT.
Hypofractionated proton beam therapy for early-stage NSCLC
Dosimetric analyses
Several treatment-planning studies have been done to compare the radiation dose that would be delivered to tumors and surrounding normal structures with PBT vs. with photon techniques for early-stage tumors. In one of the earliest analyses, investigators from the University of Florida and the Mayo Clinic assessed eight patients with medically inoperable, peripherally located lesions that had initially been treated with SABR to 48 Gy in 12 fractions. An additional set of treatment plans at the equivalent dose was then generated to identify possible differences in dose distribution to normal structures if the treatment had been passive-scattering PBT instead of SABR. The median relative difference in lung dose between the two modalities was 2-10% depending on the parameter of interest, with low-dose regions being affected more than higher doses [median difference in the volume receiving at least 5 Gy (V5) =10.4%; in V20 =2.1%; and in V40 =1.5%]; the median difference in mean lung dose was 2.2 Gy. Depending on the location of the lesion, PBT was also beneficial in other dose-volume parameters of the heart, esophagus, and bronchus. The investigators concluded from these findings that normal structure dosing was superior with PBT compared with SABR for early-stage, peripheral tumors (5).
A similar analysis done by authors from the University of Nagoya in Japan involved 21 patients with peripheral stage I NSCLC for whom plans were generated for both SABR and stereotactic body proton therapy (SBPT) to 66 Gy (RBE) in ten fractions. Again, the investigators found differences in several lung, heart, spinal cord, and esophagus doses, with the advantage from PBT again being more pronounced in the lower-dose than in the higher-dose regions in the lung. They further found that incremental increases in the tumor/target volume led to sharper rates of increase in V5 for SABR versus SBPT, but these differences were attenuated for V15-V20. Overall, because the differences in low-dose regions were more substantial when planning target volumes were larger, this group concluded that SBPT seemed to be more advantageous for larger tumors (6).
Finally, researchers at The University of Texas MD Anderson Cancer Center examined the role of SBPT for particularly challenging cases of early-stage disease, specifically tumors that were centrally or superiorly located. They compared plans for SABR, given as either passive scattering SBPT or intensity-modulated proton therapy (IMPT), for 15 patients with tumors located within 2 cm of a critical structure. They found that SABR plans could be created that would meet dose constraints for normal structures in 6 of the 15 patients, passive scattering SBPT for 12 patients, and IMPT for 14 of the 15 patients. Moreover, the proton techniques were associated with considerable improvements in target coverage when tumors were within 2 cm of the following structures: aorta, brachial plexus, heart, pulmonary vessels, and spinal cord (7) (Figure 1). Collectively, these studies demonstrated that hypofractionated PBT was dosimetrically superior to SABR for most patients with early-stage NSCLC, and that this superiority was substantially enhanced (as was the potential clinical benefit) for patients with larger, superiorly or centrally located tumors within 2 cm of a critical structure.
Clinical analyses
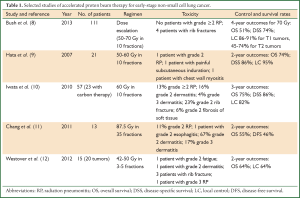
Although the sum total of clinical experience with hypofractionated PBT is still relatively limited at this time, several institutions have reported their experiences with this technique, and all showed similarly promising outcomes. These studies are summarized in Table 1. The experience with the longest follow-up comes from Loma Linda University, which has published several studies on toxicity and survival among patients with node-negative disease who underwent definitive treatment with PBT (8,13,14). In the most recent analysis, these investigators published their 12-year findings on the use of PBT to treat patients with T1-T2N0M0 peripheral NSCLC tumors (60%) or centrally located NSCLC tumors who could not undergo surgery for medical reasons or who declined resection. All patients received PBT in a dose-escalating fashion starting at 50 Gy (RBE) and increasing to 70 Gy (RBE) in ten fractions. At a median follow-up time of 48 months for the 111 patients so treated (mean tumor size, 3.6 cm), overall survival was significantly improved in patients who received 70 Gy (RBE) compared with those treated to 51 or 60 Gy (RBE) in ten fractions. Moreover, although local control rates were excellent at about 85-90% for patients with T1 tumors, the difference in control was much more significant for those with T2 lesions (4-year local control rates of 45% for those receiving 60 Gy vs. 74% for 70 Gy). Analysis of outcomes among patients who were also thought to be candidates for SABR revealed excellent rates of local control rate (96%) and overall survival (80%) at four years. Finally, treatment-related toxicity with PBT was minimal, with no patients experiencing radiation pneumonitis requiring intervention, and pulmonary function, as measured by forced expiratory volume in one second (FEV1), was largely maintained. These investigators concluded that PBT was feasible, safe, and effective for either peripheral or centrally located lesions, and that use of higher radiation doses was beneficial in terms of local control, particularly for larger tumors (8).
Full table
Other institutions have also reported outcomes with use of PBT, although the follow-up time in most studies has been shorter. Investigators from the University of Tsukuba in Japan published an initial analysis (9) and then follow-up data (15) on patients with medically inoperable stage I NSCLC treated to either 66 Gy (RBE) in ten fractions for peripherally located lesions or 72 Gy (RBE) in 22 fractions for central lesions. In the most recent report, at a median follow-up time of 17 months, the progression-free survival rates were 88.7% at two years and 78.9% at three years, with no differences found between T1 vs. T2 tumors or between central vs. peripheral lesions. Of the seven recurrences in this group of 55 patients, one was local, three were in the mediastinum or lymph nodes, and three were at other locations within the lung. Two patients experienced grade 3 pneumonitis, two grade 2, and one grade 1. One patient was noted to have a rib fracture. These investigators concluded, as did those in the Loma Linda study, that PBT was safe and feasible for patients with medically inoperable stage I disease (15).
Investigators from several institutions in Japan have reported their results PBT or carbon therapy to treat stage I NSCLC. Patients treated with PBT initially received 80 Gy (RBE) in 20 fractions, and this regimen was subsequently changed to a more aggressive alternative of 60 Gy (RBE) in ten fractions. As initially reported, at a median follow-up of approximately three years for living patients, the 3-year local control rate was 82%, with an overall survival rate at three years of 75%. Of the 80 treated patients, only one experienced grade 3 pulmonary toxicity (10). A subsequent report of outcomes among 70 patients with T2 tumors (43 treated with PBT), with the hypothesis being that control rates and toxicity would be better for this subset of patients with PBT than with SABR revealed that, at a median follow-up time of 51 months, the 4-year rates of overall survival, local control, and progression-free survival for the 70 patients were 58%, 75%, and 46%. Notably, 11 of 70 patients had mediastinal or hilar recurrences; another 12 patients with T2a or T2b tumors had similar control rates, and 2 of 70 patients experienced grade ≥3 radiation pneumonitis. Five patients had grade 3 or 4 dermatitis, and one rib fracture was reported. These investigators concluded that PBT or carbon ion therapy was well tolerated by patients with T2 disease but given the relatively high rate of distant and regional metastases, the addition of systemic therapy should be considered as well (16).
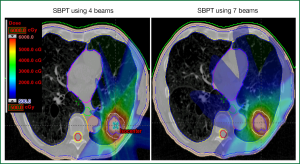
An analysis of patients treated with SBPT at Massachusetts General Hospital from 2008 through 2010 revealed a 2-year overall survival rates of 64% but a local control rate of 100% (12). Finally, in a phase I/II trial at MD Anderson Cancer Center, patients with early-stage disease who were not candidates for SABR (i.e., those with central or superior lesions or tumors >3 cm) were treated with a hypofractionated regimen of 87.5 Gy (RBE) in 35 fractions. In the first report from this trial, 18 patients had been treated at a median follow-up time of 16.3 months; no patient had experienced grade 4 or 5 toxicity, and the most common grade ≥3 adverse event was dermatitis (17%). No patient experienced grade 3 or higher pneumonitis or esophagitis. The local control rate was 89%, with 11% of patients experiencing local-regional recurrence and 28% distant metastasis. Conclusions from this study were that this regimen was well tolerated and was promising in terms of local control. Notably, the dermatitis was probably related, at least in part, to the use of two or three beams in the treatment plan (vs. using more than three beams to distribute the dose to the skin and chest wall over a larger area) (11), and thus the current practice at MD Anderson for hypofractionated regimens is to use four to six beams to minimize hot spots in that region.
Hypofractionated PBT for locally advanced NSCLC
Dosimetric analyses
Few studies to date have explored dosimetric differences between tumor targets and normal structures when hypofractionated dosing regimens are used for locally advanced disease. Therefore, such comparisons must be extrapolated from the literature on use of PBT at conventionally fractionated doses. For instance, investigators from MD Anderson Cancer Center compared dose-volume histograms in patients with stage III NSCLC treated with either PBT or (photon) IMRT and found that lung tissue parameters such as mean lung dose, V5, V10, and V20 were all improved with PBT as compared with IMRT. Doses to the lung, spinal cord, heart, and esophagus were also improved with PBT relative to IMRT (17). Similarly, a study from the University of Florida examined whether PBT could reduce the radiation dose to the lung and bone marrow [compared with 3-dimensional conformal radiation therapy (3D-CRT) or IMRT] in patients with stage III NSCLC. In plan comparisons for eight patients, PBT was associated with a median reduction of 29% in lung V20 and a 30% reduction in bone marrow V10 compared with 3D-CRT. These advantages were maintained when PBT was compared with IMRT, with PBT showing an improvement of 26% in lung V20 and 27% in bone marrow V10. In a correlative study, the same authors found that PBT could cover “high-risk” lymph nodes (mediastinal, hilar, or supraclavicular nodal regions anatomically adjacent to involved regions according to positron emission tomography) with a lung dose approximating that of photon plans that covered only involved lymph nodes, leading the authors to include that PBT could be used to expand coverage to at-risk regions without substantially increasing lung dose (18). Presumably the dosimetric advantages demonstrated in studies of locally advanced disease such as these can be extrapolated to hypofractionated therapy as well, because the proportional differences should hold with the change in fraction size.
Clinical analyses
Use of hypofractionated 3D-CRT or IMRT regimens for locally advanced disease has been evaluated by several groups; these regimens tend to involve moderate hypofractionation, with smaller fractions used than for early-stage disease because of the risks of irradiating mediastinal structures and the greater degree of lung involvement in many patients. For example, investigators from the University of Wisconsin conducted a dose-escalation study in radiation was given in 25 fractions ranging from 2.28 to 3.22 Gy. Toxicity was acceptable, with no incidences of grade ≥3 pneumonitis and 15% of patients developing grade 2 radiation pneumonitis (19). Similarly, investigators at Fudan University in Shanghai treated 34 patients with stage III NSCLC with 3D-CRT in accelerated hypofractionation, with an initial dose of 50 Gy in 20 fractions ultimately escalated to a total dose of 68 Gy after two cycles of induction chemotherapy. At three years, the median progression-free survival rate was 32% and the overall survival rate 30%, but the local-regional control rate at that time was a remarkable 61%, demonstrating that induction chemotherapy followed by hypofractionated RT is promising for such cases (20).
Another group at MD Anderson published their findings from the use of 45 Gy, delivered in 3-Gy fractions, for 26 patients with stage I-IIIB disease with involved nodes and borderline performance status, defined as a Karnofsky Performance Status (KPS) score of 60-70 or weight loss of >5%. These authors found that this regimen produced comparable survival outcomes (local control, freedom from progression) and toxicity for these patients relative to patients with higher performance status (KPS >70 and with weight loss of ≤5%) who were treated to 60-66 Gy in a standard fractionation regimen over 6 to 6.5 weeks, leading them to conclude that the accelerated treatment regimen was a reasonable alternative to conventionally fractionated doses for patients who could not tolerate concurrent chemotherapy (21). This analysis was updated after its initial publication to include 119 patients in the accelerated-treatment group and again showed no differences with regard to local or distant control compared with patients given standard fractionation regimens (22).
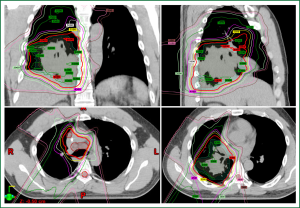
With these prior results, investigators at MD Anderson undertook the first dedicated study of hypofractionated PBT that included patients with locally advanced disease. In this phase I trial, 25 patients were treated in a dose-escalating manner with fifteen 3-, 3.5-, and 4-Gy fractions, yielding total doses of 45-60 Gy, with the dose being escalated in a 3+3 design. Thus 3 patients were treated to 45 Gy, 4 patients to 52.5 Gy, and 18 patients to 60 Gy. At a median follow-up time of 13 months for patients who were alive at the time of analysis, the authors found that only two patients had experienced dose-limiting toxicity, one with grade 3 infectious pneumonia after receiving a dose of 60 Gy in 4 Gy fractions and the other with a grade 5 tracheoesophageal fistula developing nine months after PBT to 52.5 Gy in 3.5-Gy fractions (23). However, the latter patient had also received bevacizumab, which has been shown to cause fistulas (24,25), at one month before developing the fistula. These investigators concluded that hypofractionated PBT to the thorax was well tolerated even when significant doses were delivered to the lung and central structures such as the bronchus and esophagus. This analysis also involved the development of unique dose constraints, based on extrapolations of those used in standard fractionated regimens and adjusted for biologically equivalent dose, which can be used as a foundation for future trials examining analogous regimens for mediastinal disease. Representative dose distributions for a patient treated to 60 Gy in 4 Gy fractions in that study are shown in Figure 2.
Conclusions and future directions
The feasibility of hypofractionated dose-escalated PBT for NSCLC has been demonstrated by several groups at a variety of institutions. The evidence is stronger for early-stage disease, as more studies have focused solely on PBT. The clinical benefit of PBT remains to be seen; SABR, particularly for small, peripherally located lesions, appears to produce excellent results, with local control rates often exceeding 95% and modest toxicity (1). The benefit of hypofractionated SABR in this context may be limited to patients with larger or centrally or superiorly located lesions or patients with recurrent disease. To address this possibility, investigators from MD Anderson and Massachusetts General Hospital have begun a randomized phase II study comparing SABR with SPBT for patients with centrally located stage I, selected stage II, or recurrent NSCLC (Figure 3). Candidates for this study must have primary tumors located within 2 cm of the bronchial tree, major vessels, or mediastinal structures; or T2/T3 lesions with involvement of the mediastinal pleura or pericardium; or recurrent disease. Patients are randomly assigned to receive SBRT or SBPT to a total dose of 50 Gy in four fractions, and the primary outcome is a reduction in the 2-year toxicity rate. This study will provide valuable information to address the question of whether patients with more challenging tumors would benefit more from SBRT or PBT.
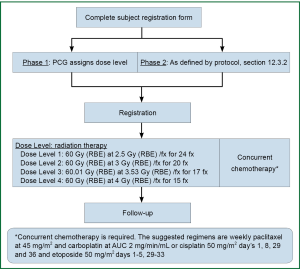
Regarding hypofractionated PBT for locally advanced disease, dosimetric analyses have shown a benefit for PBT over 3D-CRT or IMRT in select cases, and this advantage can reasonably be extrapolated to the hypofractionated context. Several phase I and phase II trials have also demonstrated the feasibility of hypofractionated regimens for patients with stage II-III disease who are not candidates for concurrent chemoradiation, with promising local control rates and acceptable toxicity. However, dose-escalation regimens in such cases have been somewhat limited by normal tissue constraints and the degree to which mediastinal structures can be spared. Ideally, the dosimetric advantages of PBT would translate into the ability to prescribe increasing fraction sizes, which would maintain reasonable rates of adverse events while improving local control. To date, only one published study has focused solely on hypofractionated PBT for NSCLC, and this analysis showed limited toxicity. However, much more information is needed regarding the safety of hypofractionated PBT before it can be widely adopted, and long-term follow-up is urgently needed to assess chronic toxicities (those appearing more than 12 months after treatment) and rates of disease control and survival compared with conventionally fractionated regimens and prior studies using photon techniques. In a phase I/II study recently opened through the Proton Cooperative Group (Figure 4), patients are to receive concurrent chemotherapy at escalating doses of hypofractionation; this regimen is intended for patients with higher performance status who are also candidates for systemic therapy. The concept is that the increased sparing of normal tissues afforded by PBT will allow more aggressive approaches to be used. Over the next several years, given the growing number of PBT facilities, collaborative efforts in prospective, ideally randomized studies will be crucial for developing appropriately individualized treatments that can take advantage of PBT, a valuable yet limited, resource-intensive, and costly modality, in the hypofractionated setting.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Langer CJ, Manola J, Bernardo P, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst 2002;94:173-81. [PubMed]
- Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer 2001;92:2639-47. [PubMed]
- Hoppe BS, Huh S, Flampouri S, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol 2010;97:425-30. [PubMed]
- Kadoya N, Obata Y, Kato T, et al. Dose-volume comparison of proton radiotherapy and stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;79:1225-31. [PubMed]
- Register SP, Zhang X, Mohan R, et al. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1015-22. [PubMed]
- Bush DA, Cheek G, Zaheer S, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: results of a 12-year experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys 2013;86:964-8. [PubMed]
- Hata M, Tokuuye K, Kagei K, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys 2007;68:786-93. [PubMed]
- Iwata H, Murakami M, Demizu Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer 2010;116:2476-85. [PubMed]
- Chang JY, Komaki R, Wen HY, et al. Toxicity and patterns of failure of adaptive/ablative proton therapy for early-stage, medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1350-7. [PubMed]
- Westover KD, Seco J, Adams JA, et al. Proton SBRT for medically inoperable stage I NSCLC. J Thorac Oncol 2012;7:1021-5. [PubMed]
- Bush DA, Hillebrand DJ, Slater JM, et al. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology 2004;127:S189-93. [PubMed]
- Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest 1999;116:1313-9. [PubMed]
- Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the university of tsukuba. Int J Radiat Oncol Biol Phys 2010;78:467-71. [PubMed]
- Iwata H, Demizu Y, Fujii O, et al. Long-term outcome of proton therapy and carbon-ion therapy for large (T2a-T2bN0M0) non-small-cell lung cancer. J Thorac Oncol 2013;8:726-35. [PubMed]
- Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:1087-96. [PubMed]
- Nichols RC, Huh SH, Hoppe BS, et al. Protons safely allow coverage of high-risk nodes for patients with regionally advanced non-small-cell lung cancer. Technol Cancer Res Treat 2011;10:317-22. [PubMed]
- Adkison JB, Khuntia D, Bentzen SM, et al. Dose escalated, hypofractionated radiotherapy using helical tomotherapy for inoperable non-small cell lung cancer: preliminary results of a risk-stratified phase I dose escalation study. Technol Cancer Res Treat 2008;7:441-7. [PubMed]
- Zhu ZF, Fan M, Wu KL, et al. A phase II trial of accelerated hypofractionated three-dimensional conformal radiation therapy in locally advanced non-small cell lung cancer. Radiother Oncol 2011;98:304-8. [PubMed]
- Nguyen LN, Komaki R, Allen P, et al. Effectiveness of accelerated radiotherapy for patients with inoperable non-small cell lung cancer (NSCLC) and borderline prognostic factors without distant metastasis: a retrospective review. Int J Radiat Oncol Biol Phys 1999;44:1053-6. [PubMed]
- Amini A, Lin SH, Wei C, et al. Accelerated hypofractionated radiation therapy compared to conventionally fractionated radiation therapy for the treatment of inoperable non-small cell lung cancer. Radiat Oncol 2012;7:33. [PubMed]
- Gomez DR, Gillin M, Liao Z, et al. Phase 1 study of dose escalation in hypofractionated proton beam therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;86:665-70. [PubMed]
- Gore E, Currey A, Choong N. Tracheoesophageal fistula associated with bevacizumab 21 months after completion of radiation therapy. J Thorac Oncol 2009;4:1590-1. [PubMed]
- Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010;28:43-8. [PubMed]


