Hyperfractionated and accelerated radiotherapy in non-small cell lung cancer
Introduction
Lung cancer is a major public health concern worldwide. Progress in improving 5-year survival is lagging behind comparable survival rates in other common cancers. Population-based lung cancer registry data analysis shows only a minimal increase in survival from 7-16% between 1995-1999 to 8-18% between 2005-2007 (1).
The majority of patients with locally advanced non-small cell lung cancer (NSCLC) are not suitable for surgical resection, often due to pre-existing co-morbidities and poor performance status. The international standard of care is concurrent chemo-radiotherapy which is associated with a 5-year survival of 20-30% and a median survival of 17-28 months (2-6). Due to the potential toxicity of concurrent chemo-radiotherapy patient selection is important. Patients with a good performance status, without major co-morbidities and assuming an acceptable radiation dose to normal tissues are eligible for this intensive treatment (7,8). Alternative treatment options are sequential chemo-radiotherapy or radiotherapy alone. Radiotherapy alone is associated with a 5-year survival of less than 5% due to local, regional and distant relapse. Local control with standard 3D conformal radiotherapy remains poor, with reported two years loco-regional control rates of 20-44% (9-11).
However, recent studies have shown that better local control of lung cancer can lead to an improvement in overall survival (10), prompting interest in altering radiotherapy delivery regimes. High dose stereotactic ablative body radiotherapy typically delivering >100 Gy biologically effective dose (BED) in 3-8 fractions is associated with very high in-field local control rates, but such doses cannot be delivered safely to locally advanced tumours due to the proximity of organs at risk such as the proximal bronchial tree, heart and spinal cord. A gap between radiation fractions allows recovery of damage in normal tissues and may also increase the sensitivity of the tumour cells to radiation by processes such as reoxygenation (12). If the individual fraction size is reduced and the fractions delivered closer together (e.g., twice daily), it may be possible to increase the dose without detriment to normal tissues.
One of the strategies to improve local control is dose escalation. Evidence gathered from the standard radiation schedules utilised in NSCLC over the past 40 years have confirmed the importance of total dose as a factor in tumour response (13). These schedules often use a single treatment of 1.8-2 Gy fractions per day over 5 days per week for a period of 5-7 weeks.
The RTOG 0617 study has evaluated dose escalation in the context of standard fractionation (2 Gy/day) and concurrent chemo-radiotherapy (5). Unfortunately the study was closed early due to futility indicating the absence of a survival benefit to high dose radiotherapy (74 Gy in 37 fractions delivered over 7.5 weeks) compared to standard dose (60 Gy in 30 fractions delivered over 6 weeks) (5).
An alternative approach to increasing the biological tumour dose in NSCLC is to develop new fractionation regimes, most commonly by hyperfractionation or acceleration. Hyperfractionation is a radiation treatment in which the total dose of radiation delivered is divided into smaller doses and treatments are given more than once a day (typically 2-3 a day). Acceleration means radiation treatment in which the total dose of radiation is given over a shorter period of time (fewer days) compared to standard radiation therapy. A recent meta-analysis by Mauguen and co-workers, evaluated ten trials including 2,000 patients and concluded that modifying the radiotherapy schedule by hyperfractionation, acceleration or both resulted in an increase in overall survival (14). The use of modified radiotherapy led to a 12% reduction in the risk of death (P=0.009). The absolute increase in overall survival in the NSCLC patients was by 3.8% at three years and 2.5% at five years, improving the survival rate from 15.9% to 19.7% at three years and from 8.3% to 10.8% at five years (14). Modified radiotherapy increased the risk of acute severe oesophagitis from 9% to 19% (P<0.001), and as expected the most accelerated regimes were associated with the most severe toxicity. However, at least 90% of patients completed the planned radiotherapy, with compliance in the experimental arms similar to the control arms. A summary of both hyperfractionation and acceleration is presented below.
Hyperfractionation
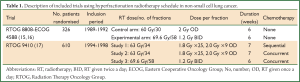
Early clinical trials evaluating hyperfractionation in the late 1980’s and early 1990’s investigated the benefit of adding chemotherapy to radiotherapy. The RTOG 8808-ECOG 4588 randomised 458 patients to two months of induction chemotherapy with cisplatin and vinblastine, followed by conventional radiotherapy (60 Gy in 2 Gy per fraction), or radiotherapy alone, with either the same radiotherapy regime or a hyperfractionated regime of 1.2 Gy per fraction delivered twice daily to a total dose of 69.6 Gy (15,16). This study showed that patients receiving induction chemotherapy did best, with a median survival of 13.2 months and a 5-year overall survival of 8% (P=0.04). Although the twice-daily radiation arm performed slightly better compared with the conventional radiation arm, the difference was not statistically significant (median survival 12 vs. 11.4 months, 5-year overall survival 6% vs. 5%).
The trials evaluating hyperfractionated radiotherapy are summarised in Table 1. One of these pivotal trials in demonstrating the advantage of concurrent over sequential chemo-radiotherapy was the RTOG 9410 study (17). It also addressed the important question of overall treatment time in the management of stage III NSCLC. This 3-arm study randomised patients to sequential chemo-radiotherapy with cisplatin/vinblastine followed by radiotherapy (60 Gy in 30 fractions of 2 Gy over six weeks) beginning on day 50 (arm 1); concurrent chemo-radiotherapy with combination cisplatin/vinblastine and the same radiotherapy beginning on day 1 (arm 2); vs. concurrent chemo-radiotherapy using combination cisplatin/etoposide with hyperfractionated radiotherapy beginning on day 1 (69.6 Gy in 58 fractions of 1.2 Gy twice daily, over six weeks) (arm 3). Phase II data suggested that the hyperfractionated regimen in arm 3 would be superior (17). However survival in the RTOG 9410 study was actually higher for patients treated with the concurrent regimen with once-daily radiotherapy (arm 2) compared with the concurrent regimen using twice-daily radiotherapy (arm 3) (P=0.046) (17). Median survival times were 14.6%, 17% and 15.6%, with five years survival of 10%, 16% and 13% for arms 1-3, respectively (P=0.046). This trial highlighted that dose escalation by a hyperfractionation regime delivered over a standard overall treatment time does not improve survival. In addition the results supported the use of concurrent chemo-radiotherapy with conventional fractionation, which has since become the gold standard treatment in good performance status stage III patients (3).
Full table
Accelerated hyperfractionation
Three fractions per day regime
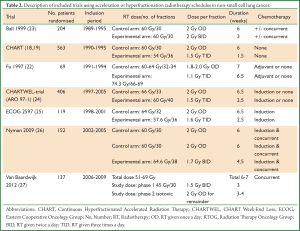
Treatment using continuous hyperfractionated accelerated radiotherapy (CHART) was shown to be of significant benefit by improving local control and overall survival (18,19). The randomised trial recruited 563 patients, PS 0-1, medically inoperable, and compared CHART (54 Gy in 36 fractions of 1.5 Gy 3 times per day over 12 consecutive days) to conventionally fractionated radiotherapy (60 Gy in 30 once daily fractions of 2 Gy over six weeks). As anticipated the main toxicity during treatment was dysphagia, which was more severe in the CHART patients, with 19% experiencing severe dysphagia, compared with 3% in the conventional group. Overall there was a 24% reduction in the relative risk of death in the CHART arm and the overall survival rates were significantly higher: 30% vs. 21% at two years and 12% vs. 7% at five years respectively for the CHART and conventional radiotherapy arm (P=0.004) (18,19). On subgroup analysis, CHART demonstrated an even greater improvement for squamous cell carcinomas, with an overall survival at three years of 21% compared with 11% for the conventional regime (P=0.0007). This evidence suggests that reducing overall treatment time in an effort to reduce tumour repopulation plays a key role in tumour control and treatment of NSCLC. Meanwhile, it should be noted that (I) the control arm of CHART would not be considered current standard of care as chemotherapy is not delivered with radiotherapy (either sequentially or concurrently) and (II) a large percentage of patients had stage I-II disease (36%) who would nowadays be considered for a surgical approach or in some cases stereotactic ablative body radiotherapy. Despite the overall benefit seen with hyperfractionated accelerated radiotherapy in the CHART trial, this has not become standard practice. Recently published data gathered from a survey of UK clinical oncologists (20), revealed 55 Gy in 20 daily fractions as the commonest fractionation schedule for NSCLC in the UK, followed by 66 Gy in 33 daily fractions. Only 14/50 centres offered CHART despite the National Institute for Health and Clinical Excellence (NICE) recommending CHART as highly cost-effective (21). It is widely recognised that the schedule is demanding for patients and requires flexible and ad hoc radiotherapy department staffing willing to work extended day. If patients are unable to travel this treatment often necessitates a 12-day inpatient stay.
Between 1991 and 1994, Fu et al. conducted a phase I/II trial evaluating hyperfractionated accelerated radiation therapy (HART) which was published as a comparative cohort study. HART was delivered by 1.1 Gy per fraction, three fractions per day at intervals of four hours with five treatment days per week (22). The clinical disease was irradiated to 74.3 Gy delivered in 66-69 fractions over 33 days (not corrected for lung density), and the subclinical disease to 50.0 Gy delivered in 44-46 fractions over 33 days. There were 60 patients in the HART group and their survival and local control results were compared to those of 50 patients treated by conventional fractionated irradiation during the same period. Survival and local control were improved in the HART group. Three-year survival was 28% vs. 6% (P<0.001). Three-year local control was 29% vs. 5% (P=0.008). Median survival for HART was 22.6 months compared with 14.0 months for standard radiotherapy patients (P<0.05).
The evolving evidence in favour of concurrent chemo-radiotherapy led to the premature closure of a number of clinical trials evaluating accelerated and hyperfractionated regimen. The trials which evaluated both these fractionation schedules as the primary treatment modality are summarised in Table 2. The ECOG 2,597 trial was closed in June 2001 when 141 patients had been recruited, reaching 42% of the overall target (25). This trial randomly assigned stage III NSCLC patients to induction chemotherapy followed by standard thoracic radiotherapy (64 Gy, 2 Gy once daily over 6.5 weeks), vs. induction chemotherapy followed by HART (57.6 Gy, 1.5 Gy in three daily fractions over 2.5 weeks, with weekend breaks). Although not statistically significant there was an improvement in survival with HART (20.3 vs. 14.9 months; P=0.28).
Full table
The CHART schedule was logistically difficult for radiotherapy departments to implement due to the additional weekend and evening treatments. This led to the CHARTWEL-trial evaluating hyper-fractionated accelerated radiotherapy which omitted weekend treatments (24). The CHARTWEL-trial compared 60 Gy in 1.5 Gy fractions, delivered 3 times per day, on the 5 weekdays, over an average of 17 days vs. conventional treatment of 66 Gy in 33 fractions delivered once daily over 45 days. The study found no significant difference between the two arms, with two years survival rates of 32% in the conventional arm and 31% in the CHARTWEL arm (P=0.43). However, this study confirmed the importance of a time factor in this disease as the lower total dose in the CHARTWEL arm was compensated by the shorter overall treatment time.
Another strategy is to dose escalate CHART. Continuous hyperfractionated accelerated radiotherapy escalated dose (CHART-ED) was a multi-centre phase I feasibility study which completed recruitment in September 2012. It compared dose-escalated CHART, adding twice daily fractions after completion of 54 Gy in 36 fractions over 12 days (28). Patients were treated on day 15 in group 1 (total dose 57.6 Gy in 38 fractions), days 15-16 in group 2 (total dose 61.2 Gy in 40 fractions) and days 15-17 in group 3 (total dose 64.8 Gy in 42 fractions). The incidence and grade of potentially dose-limiting toxicities will be assessed to determine whether dose escalation of around 6-10 Gy using this approach is safe, and the data is currently awaited.
Two fractions per day regime
An Australian study by Ball et al. used a 2×2 factorial design to evaluate shortening of the overall treatment time and the addition of carboplatin in patients with inoperable NSCLC (23). The trial randomised 204 patients between conventional radiotherapy (60 Gy in 30 fractions, once daily over six weeks) or accelerated radiotherapy (60 Gy in 30 fractions, twice daily, over three weeks) with or without concurrent carboplatin chemotherapy. Oesophageal toxicity was significantly higher in the three week radiotherapy arms and no significant survival difference between the groups was found.
Between June 2002 and May 2005 152 patients with stage III NSCLC, PS 0-1 were randomised in a Swedish 3-arm (A, B and C) phase II study by Nyman et al. (26). All arms started with two cycles of induction chemotherapy (carboplatin/paclitaxel), a third cycle was given concomitant with the start of accelerated radiotherapy in arm A (64.6 Gy in 1.7 Gy twice-daily fractions over 4.5 weeks), while in the remaining arms (B and C) conventional radiotherapy (60 Gy in 2 Gy daily fractions over 6 weeks) was combined with daily or weekly chemotherapy. Toxicity for all arms was similar and manageable with 12% grades 3-4 esophagitis, 1% grades 3-4 pneumonitis (all arms combined). Median survival was 17.8 (14.4-23.7) months (17.7, 17.7 and 20.6 months for A, B and C respectively). The 1-, 3- and 5-year overall survival was 63%, 31% and 24%. This study demonstrated that similar survival results could be achieved by intensifying treatment with either accelerated fractionated radiotherapy or concomitant chemo-radiotherapy.
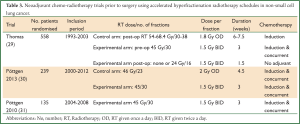
Between 1995 and 2003 the German Lung Cancer Co-operative Group (GLCCG) evaluated the role of accelerated hyperfractionated chemo-radiotherapy regimes in the pre-operative setting (29). The trials which included this fractionation schedule in the neoadjuvant setting are summarised in Table 3. 558 patients with stage IIIA-IIIB NSCLC were randomised between pre-operative chemo-radiotherapy and chemotherapy alone. In the control arm three cycles of cisplatin and etoposide chemotherapy were delivered followed by surgical resection, then adjuvant radiotherapy at 1.8 Gy daily fractions, the total dose dependent on surgical resection margins (54 Gy for negative margins, 68.4 Gy for positive margins). In the experimental arm the same induction chemotherapy was delivered, but followed by concurrent chemo-radiotherapy 45 Gy at 1.5 Gy twice daily fractions with carboplatin and vindesine, prior to surgical resection. If the margins were negative no further radiotherapy was given. But in the presence of positive margins, additional radiotherapy of 24 Gy at 1.5 Gy twice daily fractions was delivered. Pneumonectomies were performed in 35% of the patients in each group, with an increase in treatment-associated mortality seen in the experimental arm. Overall a similar number of patients underwent surgery, with a slightly higher complete resection rate in the experimental arm of 37% compared with 32% in the control arm. However there was no difference in progression free survival, the primary endpoint of this trial (29).
Full table
Pöttgen et al. also evaluated neo-adjuvant accelerated hyperfractionated chemo-radiotherapy. In an observational study, 239 patients with stage III NSCLC were treated with neoadjuvant radiochemotherapy using either accelerated hyperfractionation (45 Gy in 1.5 Gy twice-daily fractions over three weeks) or conventional fractionation (46 Gy in 2 Gy once daily fractions over 4.5 weeks) prior to thoracotomy (30). The crude pathological complete response (pCR) rates of 37% and 24% were seen in the accelerated hyperfractionated group and conventional fractionated group respectively, with a significant relationship between pCR rates and the BED suggesting an improvement in local effectiveness of accelerated hyperfractionation in lung cancer.
This accelerated regimen was further evaluated in a prospective trial by the same group in stage III NSCLC patients not deemed resectable, mainly stage IIIB (31). After three cycles of induction chemotherapy (cisplatin/paclitaxel) concurrent chemo-radiotherapy was delivered (accelerated hyperfractionated, 45 Gy in 1.5 Gy twice daily fractions over three weeks, with cisplatin/vinorelbine). Once 45 Gy was reached, a multidisciplinary panel decision was made regarding operability. Inoperable patients received definitive radiotherapy (total dose 65 or 71 Gy, depending on the mean lung dose) with additional concurrent chemotherapy (cisplatin/vinorelbine). The majority (21 of 28 patients) received 71 Gy. Oesophagitis Grade 3+ was observed in 18% and pneumonitis Grade 3+ in 4% of the patients. At three years, the loco-regional control rate was 52% (95% CI, 29-75%). In an exploratory analysis, those patients receiving 71 Gy had a loco-regional control at two and three years of 74% (95% CI: 51.2-96.3%) and 63% (95% CI: 36.1-90.4%), while in those patients receiving the lower total dose (65 Gy), loco-regional control at two and three years was 18% (95% CI: 0-49.2%; P=0.001, Wilcoxon test), respectively. Overall survival at three years was 31% (95% CI: 12-50%) for all patients. This study led to the ESPATÜ trial, a phase III multicentre study that compared induction chemotherapy followed by definitive concurrent chemo-radiotherapy to trimodality treatment (induction chemotherapy followed by concurrent chemo-radiotherapy followed by surgery). The study recently closed and results are awaited.
Given the evidence in favour of hyperfractionation and acceleration, this has been taken a step further with specifically tailored regimes. The MAASTRO group have pioneered the concept of “isotoxic” radiotherapy allowing for individualised dose escalation in stage I-III patients based on dose delivered to organs at risk (such as lung and spinal cord), using hyperfractionated accelerated radiotherapy (32). In the first MAASTRO study 166 NSCLC patients (59% stage III) not suitable for concurrent chemo-radiotherapy received an individualised dose of radiotherapy alone or after induction chemotherapy (55% of patients). Using 3D conformal therapy, the total dose delivered was between 50.4-79.2 Gy (delivered within an accelerated schedule of 1.5 Gy twice daily). With a median follow-up of 31.6 months, the median overall survival was 21.0 months—95% CI, 15.8 to 26.2 months, (stage IIIA 16.2 months—95% CI, 7.6 to 24.8 months; stage IIIB, 17.2 months—95% CI, 8.4 to 26.0 months) with a 2-year overall survival of 45.0%. Only eight patients (4.8%) developed acute grade 3 dysphagia. Less than 10% of patients with stage III received the maximum dose as per protocol of 79.2 Gy.
A further MAASTRO study, evaluated the same strategy in the concurrent setting (27), only in stage III NSCLC patients. One hundred and thirty seven patients were included in this phase II study and treated with 3D conformal radiotherapy. The individually prescribed dose was based on mean lung dose of 19 Gy, spinal cord dose of 54 Gy, brachial plexus dose of 66 Gy and central mediastinal structure dose of 74 Gy. A total dose between 51 and 69 Gy was delivered in 1.5 Gy twice daily up to 45 Gy, followed by 2 Gy once daily and radiotherapy was started at the 2nd or 3rd course of chemotherapy. The median dose was 65.0±6.0 Gy delivered in 35±5.7 days. With a median follow-up of 30.9 months, the median overall survival was 25.0 months (95% CI: 19.8-30.3 months) and 2-year overall survival 52.4%. Thirty five patients (25.5%) developed G3+ dysphagia.
It should be noted that patients in the two MAASTRO group studies were treated with 3DCRT, probably limiting individualised dose escalation. The use of Intensity Modulated Radiotherapy (IMRT) could potentially allow for further dose escalation. IMRT modulates the intensity profile of radiation delivered to the patient, permitting improved targeting of the radiation dose, and in the thorax leads to a reduction in dose to organs at risk. This could therefore lead to increased tumour control probability yet with the same normal tissue complication probability (33). A planning study by The Christie using IMRT and twice daily fractionation for stage II/III NSCLC showed that this had potential to allow a further individual dose escalation in this group of patients (34). The starting point for dose escalation in this study was 55.8 Gy in 1.8 Gy per fraction delivered twice daily. The number of fractions was then increased until one or more organ at risk (OAR) tolerance dose was exceeded or a maximum dose of 79.2 Gy (i.e., 44 fraction of 1.8 Gy BD) was reached. IMRT allowed a significant dose increase in comparison to other methods (P<0.0001) while no difference was found between 3D conformal planning and inverse planning (P=0.06).
This regime will be assessed in a UK feasibility multicentre study of isotoxic hyperfractionated accelerated radiotherapy in stage III NSCLC patients not suitable for concurrent chemo-radiotherapy (ClinicalTrials.gov Identifier: NCT01836692). If isotoxic IMRT is proven to be feasible this regimen will be compared to standard sequential chemo-radiotherapy in a national phase II “pick-the-winner” trial alongside three other dose-escalated regimens currently being evaluated in the UK.
The use of concurrent chemo-radiotherapy with accelerated hyperfractionated schedules is compromised by high rates of acute mucosal toxicity which can be challenging for both patient and clinicians, however these side effects are usually transient and resolve within a few weeks of completion of radiotherapy. The Bortfeld group have raised the interesting issue that the optimal fractionation schedule (hypofractionated vs. hyperfractionated) may depend on the OAR doses (35). For larger tumours, their model which minimizes maximum BED within a serial organ suggests hyperfractionation. Thus, accelerated hyperfractionation may eventually turn out as an ideal alternative to pure dose-escalation in locally advanced NSCLC and should deserve further evaluation within properly designed randomised trials.
Conclusions
There is significant evidence that prolonging the overall treatment time, can allow cancer stem cells to repopulate, and thus be detrimental to disease outcome (36). CHART has shown improved survival over standard radiotherapy, in patients with unresectable stage I-III NSCLC. Selected patients (with ECOG performance status 1 who do not fit the criteria for sequential or concurrent chemotherapy or patients who prefer radiotherapy only) may be considered for CHART (7,8).
Within the field of thoracic oncology evidence is emerging to suggest that an accelerated hyperfractionated radiotherapy schedule may be superior to conventional treatment. We believe that such treatment should be closely combined with other strategies in order to improve local control and survival. Dose escalation and individualised radiation doses facilitated by the use of IMRT should be combined in order to increase local control and survival. This is an exciting time for thoracic radiotherapy with these developments leading towards the goal of personalised treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127-38. [PubMed]
- Rigas JR, Kelly K. Current treatment paradigms for locally advanced non-small cell lung cancer. J Thorac Oncol 2007;2 Suppl 2:S77-85. [PubMed]
- Aupérin A, Le Péchoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006;17:473-83. [PubMed]
- Rowell NP, O’rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004;CD002140. [PubMed]
- Bradley DJ, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer. Results on radiation dose in RTOG 0617. J Clin Oncol 2013;31:abstr 7501.
- De Ruysscher D, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol 2009;20:98-102. [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65 Suppl 3:iii1-27. [PubMed]
- De Ruysscher D, Faivre-Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol 2010;28:5301-10. [PubMed]
- Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst 1991;83:417-23. [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [PubMed]
- Machtay M, Paulus R, Moughan J, et al. Defining local-regional control and its importance in locally advanced non-small cell lung carcinoma: a Radiation Therapy Oncology Group analysis. J Thorac Oncol 2012;7:716-22. [PubMed]
- Stewart FA, Dörr W. Milestones in normal tissue radiation biology over the past 50 years: from clonogenic cell survival to cytokine networks and back to stem cell recovery. Int J Radiat Biol 2009;85:574-86. [PubMed]
- Perez CA, Stanley K, Grundy G, et al. Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non-oat cell carcinoma of the lung: report by the Radiation Therapy Oncology Group. Cancer 1982;50:1091-9. [PubMed]
- Mauguen A, Le Péchoux C, Saunders MI, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol 2012;30:2788-97. [PubMed]
- Sause W, Kolesar P, Taylor S IV, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000;117:358-64. [PubMed]
- Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst 1995;87:198-205. [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet 1997;350:161-5. [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 1999;52:137-48. [PubMed]
- Prewett SL, Aslam S, Williams MV, et al. The management of lung cancer: a UK survey of oncologists. Clin Oncol (R Coll Radiol) 2012;24:402-9. [PubMed]
- Available online: http://publications.nice.org.uk/lung-cancer-cg121
- Fu XL, Jiang GL, Wang LJ, et al. Hyperfractionated accelerated radiation therapy for non-small cell lung cancer: clinical phase I/II trial. Int J Radiat Oncol Biol Phys 1997;39:545-52. [PubMed]
- Ball D, Bishop J, Smith J, et al. A randomized phase III study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non-small cell lung cancer: final report of an Australian multi-centre trial. Radiother Oncol 1999;52:129-36. [PubMed]
- Baumann M, Herrmann T, Koch R, et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol 2011;100:76-85. [PubMed]
- Belani CP, Wang W, Johnson DH, et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J Clin Oncol 2005;23:3760-7. [PubMed]
- Nyman J, Friesland S, Hallqvist A, et al. How to improve loco-regional control in stages IIIa-b NSCLC? Results of a three-armed randomized trial from the Swedish Lung Cancer Study Group. Lung Cancer 2009;65:62-7. [PubMed]
- van Baardwijk A, Reymen B, Wanders S, et al. Mature results of a phase II trial on individualised accelerated radiotherapy based on normal tissue constraints in concurrent chemo-radiation for stage III non-small cell lung cancer. Eur J Cancer 2012;48:2339-46. [PubMed]
- Available online: http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=6959
- Thomas M, Rübe C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. [PubMed]
- Pöttgen C, Eberhardt W, Graupner B, et al. Accelerated hyperfractionated radiotherapy within trimodality therapy concepts for stage IIIA/B non-small cell lung cancer: Markedly higher rate of pathologic complete remissions than with conventional fractionation. Eur J Cancer 2013;49:2107-15. [PubMed]
- Pöttgen C, Eberhardt WE, Gauler T, et al. Intensified high-dose chemoradiotherapy with induction chemotherapy in patients with locally advanced non-small-cell lung cancer-safety and toxicity results within a prospective trial. Int J Radiat Oncol Biol Phys 2010;76:809-15. [PubMed]
- van Baardwijk A, Wanders S, Boersma L, et al. Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol 2010;28:1380-6. [PubMed]
- Lievens Y, Nulens A, Gaber MA, et al. Intensity-modulated radiotherapy for locally advanced non-small-cell lung cancer: a dose-escalation planning study. Int J Radiat Oncol Biol Phys 2011;80:306-13. [PubMed]
- Warren M, Webster G, Rowbottom C, et al. PO-0961 isotoxic radiotherapy for non-small cell lung cancer: is imrt the answer? Radiother Oncol 2012;103:S378-9.
- Unkelbach J, Craft D, Salari E, et al. The dependence of optimal fractionation schemes on the spatial dose distribution. Phys Med Biol 2013;58:159-67. [PubMed]
- Koukourakis MI, Giatromanolaki A, Tsakmaki V, et al. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br J Cancer 2012;106:846-53. [PubMed]


