Review of mitral valve insufficiency: repair or replacement
Introduction
Diseases of the mitral valve (MV) are the second-most common clinically significant form of valvular defect in adults. In particular, MV regurgitation occurs with increasing frequency as part of degenerative changes in the aging process (Figure 1). The annual incidence of degenerative MV disease in industrialized nations is estimated at around 2% to 3% (1,2). In addition to degenerative changes, other causes of clinically significant MV regurgitation include cardiac ischemia, infective endocarditis and rhematic disease more frequently in less developed countries.
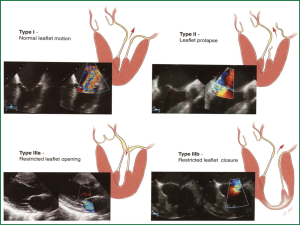
In the 1960s and 1970s, MV replacement was the highest risk adult cardiac procedure in most centers, with reported operative mortalities up to 20-30% (3). Introduction of new techniques (4), refinement of reparative methods and Carpentier’s unified approach bringing together the techniques of ring annuloplasty, leaflet reconstruction, and chordal shortening/transfer with excellent results lead the way to the establishment of MV repair as the procedure of choice in mitral insufficiency (5).
Current criteria recommend MV repair when patients develop class II symptoms, any deterioration in left ventricular function, or an end systolic diameter 4.5 cm (6,7). However, recent evidence suggests that the best outcomes after repair of severe degenerative mitral regurgitation (MR) are achieved in asymptomatic or minimally symptomatic patients, who are selected for surgery soon after diagnosis on the basis of echocardiography (2).
This review will focus on the surgical management of mitral insufficiency according to its aetiology today and will give insight to some of the perspectives that lay in the future.
Degenerative mitral incompetence
Degenerative MV disease is a common disorder affecting around 2% of the population (1). The most common finding in patients with degenerative valve disease is leaflet prolapse due to elongation or rupture of the chordal apparatus, resulting in varying degrees of MV regurgitation due to leaflet malcoaptation during ventricular contraction. Management of patients with degenerative disease revolves around the severity of regurgitation and its impact on clinical status, ventricular function and dimension, the consequences of systolic flow reversal such as atrial dilatation/fibrillation and secondary pulmonary hypertension, and the risk of sudden death (1,2,8,9).
Whether early surgical intervention in asymptomatic patients, before the onset of ventricular changes, improves the outcome of patients with chronic severe degenerative MV disease remains controversial (6,9-11).
Not all degenerative valve disease involved giant excess tissue as originally proposed by Barlow in the 1960’s (12,13). In fibroelastic deficiency adjacent leaflet segments, are usually normal or even thinned out with a translucent quality, and are of normal size and height (14-16). The valve annular size is generally normal (28-32 mm) (Figure 2A) (17).
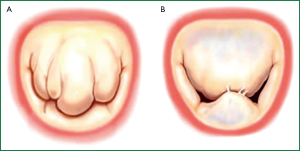
Barlow’s disease is characterized by diffuse excess tissue (Figure 2B) (14-18). Valve size is generally large, and multiple segments are usually affected with myxomatous pathological changes, the leaflets are thickened and distended. Diffuse chordal elongation in addition to chordal rupture is the rule as opposed to a simple isolated chordal rupture. Typically the valve size corresponds to a mitral ring size of ≥36 mm. Severe annular dilatation, varying degrees of annular calcification and subvalvular fibrosis and calcification of the papillary muscles may occur (18).
Patients with chronic severe MV regurgitation are usually referred for surgical intervention after the occurrence of symptoms, declining LV function, significant LV enlargement, or the development of severe pulmonary hypertension (19,20). Atrial fibrillation is a more controversial indication for surgical referral, but if present at the time of surgery, patients should probably also undergo a concomitant modified Maze procedure utilizing cryothermy or radiofrequency (21,22). Persistent atrial fibrillation after MV repair is associated with long-term morbidity including stroke as well as mortality (23,24).
Lately, earlier surgical intervention has been proposed, but controversy exists whether asymptomatic patients with severe MR and normal LV function should undergo elective MV repair (6,9,10,25-27).
Valve repair in patients with degenerative MV disease is associated with an improved quality of life with less morbidity as well as better long-term survival as opposed to replacement (28-31). Prosthesis-related morbidity, including higher re-operation rates, and the need for aggressive anticoagulation account for this difference. However, even in developed countries, MV replacement in the setting of degenerative disease remains frequent. Gammie et al. (32) reported that the repair rate for 47,126 patients with isolated MR in the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database rose from 51% to 69% between January 2000 and December 2007. In the Euro Heart Survey similar repair rates were documented around 50%. MV replacement continues to be performed far too frequently in the modern era of reconstructive valve surgery (33).
MV repair for degenerative disease follows two fundamental principles: I. Restore a good surface of leaflet coaptation and II. Correct for annular dilatation. A leaflet coaptation line of 5-8 mm is considered essential to provide a durable repair result. Intraoperative transoesophageal 2D and increasingly real time 3D transoesophageal echocardiography is applied to guide the procedure and confirm a good result (34,35).
Carpentier’s techniques which generally involve resection of abnormal or pathologic tissue with precise reconstruction toward ‘normal valve anatomy’ remain the most commonly performed world-wide, and are associated with excellent long-term outcomes (Figure 3) (5,13,36-38). Isolated prolapse of the posterior leaflet is treated by a limited triangular or quadrangular leaflet resection, including the respective elongated or ruptured chordae. The remnant leaflet margins are then readapted using interrupted sutures. For larger resections of abnormal tissue such as seen in Barlow’s disease, the annulus may be compressed with additional interrupted sutures to narrow its circumference resulting in less tension on residual reconstructed leaflet segments (10). It is imperative to avoid “overcorrection” which results in systolic anterior motion of the anterior leaflet (39,40). Transfer of secondary chords to the free margin of the same segment, or chordal transposition from one segment to another is performed when residual prolapse remains despite tailored resections of abnormal tissue.

A new concept of ‘respect rather than resect’ tissue has become popular in recent years. It is based on the use of polytetrafluoroethylene (PTFE) neochordae to reconstruct support of the free edge of prolapsing segments, and to ‘displace’ abnormal excess tissue into the ventricle ensuring a good surface of coaptation (Figure 4) (41,42). The simplicity of the technique is particularly important in small access incisions where more advanced Carpentier techniques may prove challenging.
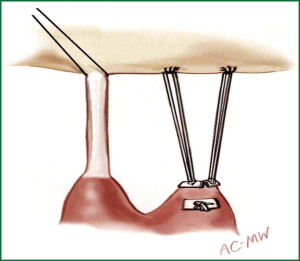
Regardless of the leaflet and chordal techniques employed, a prosthetic ring or band annuloplasty is mandatory for all repair procedures (37,43,44).
Failure to perform an annuloplasty at the time of MV repair is a predictor of failure resulting in recurrent moderate or severe mitral valve regurgitation (45).
In contemporary practice, MV replacement for degenerative disease in a primary operation should be rare. Current reports document repair rates greater than 90%, regardless of lesions or associated leaflet dysfunction (46-48).
If valve replacement is necessary, a chordal sparring procedure should be performed to maintain annular-papillary continuity (49).
Freedom from re-operation is very low in degenerative MV surgery. In a number of studies a return of moderate to severe MR has been noted in 1-2% of patients per year during mid-term follow-up (36,45,50-52).
Long-term survival following MV repair is similar to age matched controls if the operation is done before the onset of symptoms, ventricular dysfunction or atrial fibrillation (28,29,53,54).
Ischemic mitral incompetence (IMI)
IMI is the most frequent mechanism of MR today, particularly in developed countries where rheumatic MV disease has been nearly eradicated. It has poor prognosis and involves global and regional left ventricular remodelling as well as dysfunction and distortion of the MV, including the chordae, annulus, and leaflets. Ischemic MR is characterized by restrictive mitral leaflet mobility due to dyskinesia or even akinesia of the ventricular wall which bears one or both papillary muscles, thus, extending the distance between the ventricular wall and the leaflets. The posterior papillary muscle and its supporting ventricular wall and the posterior-inferior wall of the left ventricle are most frequently affected. IMI can be permanent following myocardial infarction, myocardial scarring, or even after the development of an aneurysm; however, IMI can also be transient in ischemia and hibernating myocardium (55).
It remains unclear whether patients with IMI of grade 2-3 and impaired LV function should undergo MV surgery concomitant with coronary revascularization, or simply isolated revascularization. A recent randomized trial addressing this issue in patients with ejection fraction less than 30% was stopped prematurely after showing benefit with the addition of repair to revascularization and concluded that adding mitral annuloplasty to CABG in patients with moderate ischemic MR may improve functional capacity, left ventricular reverse remodeling, MR severity, and B-type natriuretic peptide levels, compared with CABG alone. The impact of these benefits on longer term clinical outcomes remains to be defined (56).
Historically, the surgical approach to patients with functional MR of IMI was to perform MV replacement but the consequences that interruption of the annulus—papillary muscle continuity had on LV systolic function were not well understood. This procedure was associated with prohibitive mortality rates. Techniques of MV replacement, such as prosthesis implantation with preservation of the subvalvular apparatus (57), and prosthesis implantation with preservation of one or both leaflets (usually the posterior leaflet) have evolved to improve the long-term hemodynamic function and clinical status of these patients. A number of studies demonstrated that preservation of the annulus—papillary muscle continuity is of paramount importance to preservation of LV function (58,59). It was the excision and disruption of the subvalvular apparatus that was responsible for the significant loss of systolic function that led to the poor outcome in the earlier patients who underwent MV replacement (60). Replacement should be reserved for cases of acute papillary muscle rupture in relation to an acute myocardial infarction, where a large area of the ventricle is infarcted. Reimplanting the ruptured papillary muscle to an infarcted area of the ventricle might eventually lead to a repeated rupture; thus, replacing the valve remains the best alternative.
The primary goal in MV repair is to achieve complete and rapid closure of the mitral orifice by a well mobile anterior leaflet and a sufficiently large coaptation area by bringing the posterior leaflet closer to the anterior leaflet. Current surgical options for repair of the MV apparatus, e.g., quadrangular resection of the posterior leaflet with or without sliding annuloplasty, triangular resection of the middle scallop of the anterior leaflet, chordal transfer or transposition, papillary muscle shortening or reimplantation, edge-to-edge leaflet approximation, may be performed in combination with ring annuloplasty using flexible or rigid circular rings and posterior annuloplasty bands or partial rings. However, an undersized annuloplasty (Figure 5) is usually the treatment of choice for patients with IMR and dilated cardiomyopathy.
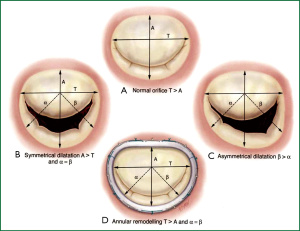
In appropriately selected patients, a well-performed restrictive annuloplasty is associated with low operative mortality and is effective in eliminating MR, promoting left ventricular reverse remodeling, reducing symptoms, and improving quality of life. Patient selection is crucial. It has been demonstrated that the ideal candidate for annuloplasty alone is a patient in the early stage of the disease, with a short history of heart failure, and a left ventricle not excessively dilated (61,62). When the tethering of the leaflets is severe (as typically occurs in patients with a long history of congestive heart failure and advanced left ventricular remodeling), residual/recurrent MR can frequently occur. Such an event has been reported in 20-30% of the patients one year after surgery and is strictly related with an inferior outcome in terms of heart failure and mortality during follow-up (63,64). Therefore, it is extremely important to avoid either residual or recurrent MR. When the preoperative clinical and echocardiographic data suggest that annuloplasty alone is unlikely to be successful and durable, additional surgical procedures should be used to enhance the effectiveness of MV repair.
Surgical mortality in patients with ischemic cardiomyopathy aged >60 years has been reported to be between 10% and 48% (65).
It is still debated whether patients with grade 2 IMI should undergo combined CABG or CABG alone. It is perceived that combined surgery improves LV function significantly, as a result of the reperfusion of ischemic myocardial areas, stabilization of the mitral annulus, and decreased volume overload secondary to MR correction.
Studies by Hausmann et al. (66,67) showed that residual MR of grade 1 or above is a strong predictor for poor survival. Prifti et al. (68) demonstrated that grade 2 MR is a strong predictor for poor overall survival in end-stage coronary artery disease patients. Czer et al. (69) showed that concomitant annuloplasty and CABG significantly reduced regurgitation by re-establishing a more normal relationship between the leaflet and annulus sizes, whereas the reduction in regurgitation grade with revascularization alone was infrequent. On the other hand, Christenson et al. (70) reported good survival and morbidity in patients with poor LV function and MVR of grade 2 undergoing CABG alone and demonstrated MVR normalization postoperatively. Likewise, Pinson et al. (71) presented a high estimated 5-year survival with moderate MVR and normal LV function in patients undergoing CABG alone. However, Duarte et al. (72) reported only “acceptable” outcome in his series of 58 patients with moderate ischemic MVR undergoing isolated CABG.
Rhematic disease
Rheumatic heart valve disease (RHVD) (Figure 6) is the result of rheumatic fever (RF) triggered by autoimmune humoral and cellular responses (73,74) and remains the predominant heart valve disease in developing countries (73-78). It is seen in epidemic proportions in the preschool and school age groups but is also found in patients in their teens and early twenties. A number of patients present during adulthood at a mean age of 40 years in developing countries and in their early 50s and 60s in developed countries (76-84). RHVD has become rare, virtually nonexistent in most developed countries (75), but remains uncontrolled in still developing countries with an incidence of 1.6/1,000 in Liberia, 2.2/1,000 in Cambodia, and 2.3/1,000 in Mozambique, as determined by clinical diagnosis without echocardiograhic support (76,77). The global burden is estimated to be 15.6 million and about 282,000 new cases are registered each year with an annual mortality of 233,000 (78).

Until recently, MV replacement was the only surgical option for patients with a severely diseased MV. Carpentier advocated surgical repair of the diseased MV apparatus to restore the interaction between the papillary muscle, the chordae and leaflets and the MV function (5).
Options for the repair of rheumatic MV disease include the following: (I) synthetic or autologous pericardial strip annuloplasty (PSA) for annular disease; (II) posterior plication annuloplasty suture; (III) leaflet remodeling by resection and sliding plasty of the posterior annulus or plication of the involved posterior leaflet; (IV) leaflet augmentation with autologous pericardium (AP) for leaflet disease; (V) leaflet thinning (removal of fibrous tissue around the cusp); (VI) partial replacement of the MV apparatus with a segment of an autologous tricuspid valve apparatus; (VII) incision of fused commissural chordae; (VIII) open mitral commissurotomy with or without papillary muscle split; (IX) resection of secondary chordae; (X) shortening of elongated chordae; (XI) transposition of elongated chordae; (XII) expanded polytetrafluoroethylene (ePTFE, Gore-Tex®) artificial chordal replacement (ACR) for chordal disease.
The procedure especially benefits patients from a poor social background without health insurance because it would curtail the cost of long-term anticoagulation and frequent hospital visits for monitoring the international normalized ratio (INR) (81,82,85,86). In regions where the life expectancy is less than 60 years, repair of the native valve is preferred as an option to obviate serious valve-related complications of mechanical prosthesis, e.g., thromboembolic episodes and sudden death. Bioprostheses are an alternative device for replacement of a diseased MV that is beyond repair. Bioprostheses are known to develop early structural valve deterioration and, thus, have limited durability in younger age groups (87-89).
Results of anterior mitral leaflet (AML) augmentation with autologous pericardium in Acar’s series (90) have demonstrated the role of this technique in a subset of patients—in particular, in children with retracted deficient leaflet tissue. The rate of reoperation for leaflet augmentation versus no augmentation was 2.5% versus 12.9%, respectively, at 2.8 years of follow-up (P<0.05) (90).
Failures resulting in a mild MR, as evidenced by intraoperative transesophageal echocardiography, which occur within 2-3 years after repair are related to improper indication, inadequate repair, and technical factors (91-98). Consequently, to avoid early reoperation, an inadequate leaflet coaptation of less than 8 mm in length with a mild MR in the operating room should not be accepted. Reoperations that occur beyond seven years were found to be due to recurrent rheumatic activity, which leads to a progressive structural deterioration of the mitral apparatus. Regular postoperative echocardiographic and clinical studies and penicillin prophylaxis in endemic regions are highly recommended (9,10,19).
Limited data are available to compare late outcomes after rheumatic mitral repair versus valve replacement. In the study by Yau and associates (93), 25% of 573 patients with rheumatic mitral disease had repair, and after risk adjustment with a Cox model, operative mortality was better with repair (0.7% after repair vs. 5.1% for replacement) as was late survival. Valve-related complications were lower after repair, although late reoperation was higher. At a mean follow-up of 68 months, 16% of repair patients required reoperation, although no reoperative mortalities occurred. These data suggest a survival benefit of mitral repair for rheumatic disease, albeit with a higher reoperation rate.
Infective endocarditis
In the mid 1960s, valve replacement was proposed for patients with MV endocarditis by Robicsek and coauthors (99). In 1990, Dreyfus et al. (100) were the first to demonstrate the feasibility of MV repair in active infective endocarditis (AIE), introducing the concept of early surgery to prevent further destruction of the valve. Others have also confirmed the feasibility of MV repair and better survival than with MV replacement in patients undergoing surgery for either active or healed endocarditis (101,102). However, these observations, which are based on data derived from studies with small sample sizes and limited follow-ups, are mostly not comparable with each other because of their heterogeneity (103-105).
Surgical strategy as described by R. Hetzer includes three principles that should be kept in mind when operating for infective endocarditis:
I. Intensive and wide debridement of all macroscopically involved tissue without concern for the possibility of repair. Excision of a vegetation alone is limited to patients with a well-circumscribed vegetation and a well-defined shaft in an otherwise normal valve. If the vegetation has a wide base and no well-defined shaft, the base is also excised.
II. Whenever possible, valve defects which emerge from vegetectomy are repaired with homologous or autologous pericardium (Figure 7) using monofilament sutures reinforced with horse pericardium and preserved in polyvidone-iodine solution. To ensure leaflet coaptation, annuloplasty with pericardium is performed. In order to avoid artificial material in an infected field, no prosthetic ring annuloplasty device is used.
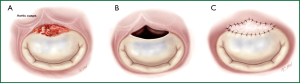
III. If valve replacement is unavoidable because of extensive endocarditic destruction of the leaflets or due to the poor quality of the remaining tissue, MV replacement is performed.
It has been shown that MV repair for AIE can be performed not only with low operative mortality and satisfactory early and long-term survival, but also with excellent freedom from recurrent infection and repeat operation. There are well-accepted class 1 grade A level of evidence preferring MV repair over MV replacement (106). If MV repair is feasible, it has been shown to reduce operative mortality and improve long-term survival and functional status in the comparison with MV replacement (107).
Future perspectives
Minimally invasive techniques
In recent years the minimally invasive procedure via a right lateral minithoracotomy with femoral catheterization (i.e., the heart-lung machine is connected via the femoral vessels) has become established (50). The safety and efficacy of this operation have been shown in large series (50,108-110). Depending on the surgeon’s experience, minimally invasive reconstruction rates for patients with MR as their main disease are over 80%, and for isolated MR they are as high as 97% (50,110,111). Long-term survival, at over 82% at five years (Kaplan-Meier analysis), is comparable to that of the normal population. These patients also have very low reoperation rates, with nearly 97% success at five years (51,112).
Large series confirm unequivocally the quality and reproducibility of this operation (108,113,114). Advantages of this method are considered to be the very small wound surface area, rapid recovery, reduced postoperative pain, and better cosmetic result (Figure 8) (115).
Robotic MV surgery
The first robotic MV repair was performed in May 1998 by Dr. Carpentier using an early prototype of the da Vinci® articulated intracardiac “wrist” robotic device (116). A week later, Dr. Mohr performed the first coronary anastomosis and repaired five MVs with the device (117). It is advocated that the dexterity of the robotic arms allows the surgeon to precisely place sutures in locations that were routinely difficult to reach (e.g., left trigone) when using sternotomy or minimally invasive-based approaches.
Dr. Murphy et al. (118) reported their experience in 127 patients; seven patients had valve replacement and 114 had repair. There was one inhospital death, one late death, two strokes, and 22 patients developed new onset of atrial fibrillation. Blood product transfusion was required in 31% of patients and 2 (1.7%) patients required reoperation. Post discharge echocardiograms were available in 98 patients at a mean follow-up of 8.4 months with no more than 1+ residual MR in 96.2%. Chitwood et al. reported on 300 patients undergoing robotic MV repair between May 2000 and November 2006 having echocardiographic and survival follow-up in 93% and 100% of patients, respectively (119). There were 2 (0.7%) 30-day mortalities and 6 (2.0%) late mortalities. Complications included 2 (0.7%) strokes, 2 transient ischemic attacks, 3 (1.0%) myocardial infarctions, and 7 (2.3%) reoperations for bleeding. The mean hospital stay was 5.2±4.2 (standard deviation) days. A total of 16 (5.3%) patients required reoperation. Echocardiographic follow-up demonstrated the following degrees of MR: none/trivial, 192 (68.8%); mild, 66 (23.6%); moderate, 15 (5.4%); and severe, 6 (2.2%).
The introduction of newer robotic instrumentation like the dynamic left atrial retractor and simpler MV repair techniques (120) may facilitate the use of robotic MV techniques by a larger number of cardiac surgeons.
Conclusions
In the developed countries, where mitral incompetence of degenerative and ischemic origin is the rule most cases of MV disease can be successfully approached by repair. Rheumatic valve disease still exists in areas with less developed health care systems. Repair in rheumatic valve disease has been advocated, but still, in this disease a certain proportion of chronic cases with calcification may require valve replacement. Similarly, in AIE, repair again may be unsuccessful. However, it may be attempted even with some residual incompetence, accepting that re-operation may become necessary at a time when the infection has been cured.
Enthusiasm exists for less invasive approaches to the MV, either by small chest incisions or even with the use of robotic techniques. The exact role of these procedures remains to be determined. Currently, mitral repair is associated with less than a 1% operative mortality in many centres, and late results continue to improve. The development of effective autologous reparative procedures for the treatment of MV disease has been deemed as a success.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009;373:1382-94. [PubMed]
- Anders S, Said S, Schulz F, et al. Mitral valve prolapse syndrome as cause of sudden death in young adults. Forensic Sci Int 2007;171:127-30. [PubMed]
- Kouchoukos NT. Problems in mitral valve replacement. In: Kirklin TW. eds. Advances in Cardiovascular Surgery. Grune & Stratton, New York,1973:205-16.
- Rankin JS. Mitral and tricuspid valve disease: Historical aspects. In: Sabiston DC, Jr. eds. Textbook of Surgery. WB Saunders Company, Philadelphia, 1986:2345.
- Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Rosenhek R, Rader F, Klaar U, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation 2006;113:2238-44. [PubMed]
- Adams DH, Anyanwu AC. Seeking a higher standard for degenerative mitral valve repair: begin with etiology. J Thorac Cardiovasc Surg 2008;136:551-6. [PubMed]
- Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol 2008;52:319-26. [PubMed]
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [PubMed]
- Kang DH, Kim JH, Rim JH, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation 2009;119:797-804. [PubMed]
- Montant P, Chenot F, Robert A, et al. Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: a propensity score-based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg 2009;138:1339-48. [PubMed]
- Barlow JB, Pocock WA. The significance of late systolic murmurs and mid-late systolic clicks. Md State Med J 1963;12:76-7. [PubMed]
- Carpentier A, Lacour-Gayet F, Camilleri J. Fibroelastic dysplasia of the mitral valve:an anatomical and clinical entity. Circulation 1982;3:307.
- Anyanwu AC, Adams DH. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg 2007;19:90-6. [PubMed]
- Carpentier A, Chauvaud S, Fabiani JN, et al. Reconstructive surgery of mitral valve incompetence: ten-year appraisal. J Thorac Cardiovasc Surg 1980;79:338-48. [PubMed]
- Fornes P, Heudes D, Fuzellier JF, et al. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: a histomorphometric study of 130 excised segments. Cardiovasc Pathol 1999;8:81-92. [PubMed]
- Barlow JB, Pocock WA. Billowing, floppy, prolapsed or flail mitral valves? Am J Cardiol 1985;55:501-2. [PubMed]
- Carpentier AF, Pellerin M, Fuzellier JF, et al. Extensive calcification of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg 1996;111:718-29; discussion 729-30. [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1-142. [PubMed]
- Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68. [PubMed]
- Lee AM, Melby SJ, Damiano RJ Jr. The surgical treatment of atrial fibrillation. Surg Clin North Am 2009;89:1001-20. [PubMed]
- Lee R, Kruse J, McCarthy PM. Surgery for atrial fibrillation. Nat Rev Cardiol 2009;6:505-13. [PubMed]
- Eguchi K, Ohtaki E, Matsumura T, et al. Pre-operative atrial fibrillation as the key determinant of outcome of mitral valve repair for degenerative mitral regurgitation. Eur Heart J 2005;26:1866-72. [PubMed]
- Itoh A, Kobayashi J, Bando K, et al. The impact of mitral valve surgery combined with maze procedure. Eur J Cardiothorac Surg 2006;29:1030-5. [PubMed]
- Adams DH, Anyanwu AC. Valve disease: asymptomatic mitral regurgitation: does surgery save lives? Nat Rev Cardiol 2009;6:330-2. [PubMed]
- Detaint D, Iung B, Lepage L, et al. Management of asymptomatic patients with severe non-ischaemic mitral regurgitation. Are practices consistent with guidelines? Eur J Cardiothorac Surg 2008;34:937-42. [PubMed]
- Schaff HV. Asymptomatic severe mitral valve regurgitation: observation or operation? Circulation 2009;119:768-9. [PubMed]
- David TE, Ivanov J, Armstrong S, et al. Late outcomes of mitral valve repair for floppy valves: Implications for asymptomatic patients. J Thorac Cardiovasc Surg 2003;125:1143-52. [PubMed]
- Enriquez-Sarano M, Schaff HV, Orszulak TA, et al. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation 1995;91:1022-8. [PubMed]
- Gillinov AM, Blackstone EH, Nowicki ER, et al. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg 2008;135:885-93, 893.e1-2.
- Suri RM, Schaff HV, Dearani JA, et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg 2006;82:819-26. [PubMed]
- Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2009;87:1431-7; discussion 1437-9. [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [PubMed]
- Adams DH, Anyanwu AC, Sugeng L, et al. Degenerative mitral valve regurgitation: surgical echocardiography. Curr Cardiol Rep 2008;10:226-32. [PubMed]
- O’Gara P, Sugeng L, Lang R, et al. The role of imaging in chronic degenerative mitral regurgitation. JACC Cardiovasc Imaging 2008;1:221-37. [PubMed]
- Braunberger E, Deloche A, Berrebi A, et al. Very long-term results (more than 20 years) of valve repair with carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation 2001;104:I8-11. [PubMed]
- Carpentier A, Deloche A, Dauptain J, et al. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg 1971;61:1-13. [PubMed]
- Filsoufi F, Carpentier A. Principles of reconstructive surgery in degenerative mitral valve disease. Semin Thorac Cardiovasc Surg 2007;19:103-10. [PubMed]
- Jebara VA, Mihaileanu S, Acar C, et al. Left ventricular outflow tract obstruction after mitral valve repair. Results of the sliding leaflet technique. Circulation 1993;88:II30-4. [PubMed]
- Mihaileanu S, Marino JP, Chauvaud S, et al. Left ventricular outflow obstruction after mitral valve repair (Carpentier’s technique). Proposed mechanisms of disease. Circulation 1988;78:I78-84. [PubMed]
- Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg 2008;136:1205; discussion 1205-6. [PubMed]
- Seeburger J, Kuntze T, Mohr FW. Gore-tex chordoplasty in degenerative mitral valve repair. Semin Thorac Cardiovasc Surg 2007;19:111-5. [PubMed]
- Carpentier AF, Lessana A, Relland JY, et al. The “physio-ring”: an advanced concept in mitral valve annuloplasty. Ann Thorac Surg 1995;60:1177-85; discussion 1185-6. [PubMed]
- Gillinov AM, Cosgrove DM 3rd, Shiota T, et al. Cosgrove-Edwards Annuloplasty System: midterm results. Ann Thorac Surg 2000;69:717-21. [PubMed]
- Flameng W, Herijgers P, Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation 2003;107:1609-13. [PubMed]
- Adams DH, Anyanwu AC, Rahmanian PB, et al. Large annuloplasty rings facilitate mitral valve repair in Barlow’s disease. Ann Thorac Surg 2006;82:2096-100; discussion 2101. [PubMed]
- Gammie JS, Bartlett ST, Griffith BP. Small-incision mitral valve repair: safe, durable, and approaching perfection. Ann Surg 2009;250:409-15. [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [PubMed]
- David TE, Burns RJ, Bacchus CM, et al. Mitral valve replacement for mitral regurgitation with and without preservation of chordae tendineae. J Thorac Cardiovasc Surg 1984;88:718-25. [PubMed]
- David TE. Outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Semin Thorac Cardiovasc Surg 2007;19:116-20. [PubMed]
- David TE, Ivanov J, Armstrong S, et al. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg 2005;130:1242-9. [PubMed]
- Flameng W, Meuris B, Herijgers P, et al. Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg 2008;135:274-82. [PubMed]
- Enriquez-Sarano M, Tajik AJ, Schaff HV, et al. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 1994;90:830-7. [PubMed]
- Enriquez-Sarano M, Tajik AJ, Schaff HV, et al. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: results and clinical implications. J Am Coll Cardiol 1994;24:1536-43. [PubMed]
- Hetzer R, Delmo Walter EM. Mitral valve repair for ischemic mitral incompetence. In: Hetzer R, Rankin JS, Yankah CA. eds. Mitral Valve Repair. Springer-Verlag Berlin Heidelberg, 2011:176.
- Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012;126:2502-10. [PubMed]
- Hetzer R, Drews T, Siniawski H, et al. Preservation of papillary muscles and chordae during mitral valve replacement: possibilities and limitations. J Heart Valve Dis 1995;4 Suppl 2:S115-23. [PubMed]
- David TE, Uden DE, Strauss HD. The importance of the mitral apparatus in left ventricular function after correction of mitral regurgitation. Circulation 1983;68:II76-82. [PubMed]
- Sarris GE, Cahill PD, Hansen DE, et al. Restoration of left ventricular systolic performance after reattachment of the mitral chordae tendineae. The importance of valvular-ventricular interaction. J Thorac Cardiovasc Surg 1988;95:969-79. [PubMed]
- Huikuri HV. Effect of mitral valve replacement on left ventricular function in mitral regurgitation. Br Heart J 1983;49:328-33. [PubMed]
- Hetzer R, Delmo Walter EM. Repair of congenital mitral valve insufficiency. Oper TechThorac Cardiovasc Surg A Comparative Atlas 2010;15:260-72.
- Bolling SF. Mitral valve reconstruction in the patient with heart failure. Heart Fail Rev 2001;6:177-85. [PubMed]
- Gerbode FL, Hetzer R, Krebber HJ. Surgical management of papillary muscle rupture due to myocardial infarction. World J Surg 1978;2:791-6. [PubMed]
- Duarte IG, Murphy CO, Kosinski AS, et al. Late survival after valve operation in patients with left ventricular dysfunction. Ann Thorac Surg 1997;64:1089-95. [PubMed]
- Jones EL, Weintraub WS, Craver JM, et al. Interaction of age and coronary disease after valve replacement: implications for valve selection. Ann Thorac Surg 1994;58:378-84; discussion 384-5. [PubMed]
- Hausmann H, Siniawski H, Hotz H, et al. Mitral valve reconstruction and mitral valve replacement for ischemic mitral insufficiency. J Card Surg 1997;12:8-14. [PubMed]
- Hausmann H, Siniawski H, Hetzer R. Mitral valve reconstruction and replacement for ischemic mitral insufficiency: seven years’ follow up. J Heart Valve Dis 1999;8:536-42. [PubMed]
- Prifti E, Bonacchi M, Frati G, et al. Early and midterm outcome of coronary artery bypass grafting in endstage coronary artery disease patients. Cor Europaeum 2000;8:93-9.
- Czer LS, Maurer G, Bolger AF, et al. Revascularization alone or combined with suture annuloplasty for ischemic mitral regurgitation. Evaluation by color Doppler echocardiography. Tex Heart Inst J 1996;23:270-8. [PubMed]
- Christenson JT, Simonet F, Bloch A, et al. Should a mild to moderate ischemic mitral valve regurgitation in patients with poor left ventricular function be repaired or not? J Heart Valve Dis 1995;4:484-8; discussion 488-9. [PubMed]
- Pinson CW, Cobanoglu A, Metzdorff MT, et al. Late surgical results for ischemic mitral regurgitation. Role of wall motion score and severity of regurgitation. J Thorac Cardiovasc Surg 1984;88:663-72. [PubMed]
- Duarte IG, Shen Y, MacDonald MJ, et al. Treatment of moderate mitral regurgitation and coronary disease by coronary bypass alone: late results. Ann Thorac Surg 1999;68:426-30. [PubMed]
- Binotto M, Guilherme L, Tanaka A. Rheumatic Fever. Images Paediatr Cardiol 2002;4:12-31. [PubMed]
- Guilherme L, Ramasawmy R, Kalil J. Rheumatic fever and rheumatic heart disease: genetics and pathogenesis. Scand J Immunol 2007;66:199-207. [PubMed]
- Ayoub EM. Resurgence of rheumatic fever in the United States. The changing picture of a preventable illness. Postgrad Med 1992;92:133-6, 139-42. [PubMed]
- Yankah AC, Marshall R, Van Reken D, et al. Cardiovascular diseases in Liberia. Cardiologie Tropicale, Tropical cardiology 1981:7:15-20.
- Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med 2007;357:470-6. [PubMed]
- Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005;5:685-94. [PubMed]
- Antunes MJ, Magalhaes MP, Colsen PR, et al. Valvuloplasty for rheumatic mitral valve disease. A surgical challenge. J Thorac Cardiovasc Surg 1987;94:44-56. [PubMed]
- Bernal JM, Rabasa JM, Olalla JJ, et al. Repair of chordae tendineae for rheumatic mitral valve disease. A twenty-year experience. J Thorac Cardiovasc Surg 1996;111:211-7. [PubMed]
- Kumar AS, Talwar S, Saxena A, et al. Results of mitral valve repair in rheumatic mitral regurgitation. Interact Cardiovasc Thorac Surg 2006;5:356-61. [PubMed]
- Talwar S, Rajesh MR, Subramanian A, et al. Mitral valve repair in children with rheumatic heart disease. J Thorac Cardiovasc Surg 2005;129:875-9. [PubMed]
- Phan KP. Mitral valve repair in children using Carpentier’s techniques. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 1999;2:111-120. [PubMed]
- Ho HQ, Nguyen VP, Phan KP, et al. Mitral valve repair with aortic valve replacement in rheumatic heart disease. Asian Cardiovasc Thorac Ann 2004;12:341-5. [PubMed]
- Kim JB, Kim HJ, Moon DH, et al. Long-term outcomes after surgery for rheumatic mitral valve disease: valve repair versus mechanical valve replacement. Eur J Cardiothorac Surg 2010;37:1039-46. [PubMed]
- Skoularigis J, Sinovich V, Joubert G, et al. Evaluation of the long-term results of mitral valve repair in 254 young patients with rheumatic mitral regurgitation. Circulation 1994;90:II167-74. [PubMed]
- Alsoufi B, Manlhiot C, McCrindle BW, et al. Results after mitral valve replacement with mechanical prostheses in young children. J Thorac Cardiovasc Surg 2010;139:1189-96, 1196.e1-2.
- Alsoufi B, Manlhiot C, McCrindle BW, et al. Aortic and mitral valve replacement in children: is there any role for biologic and bioprosthetic substitutes? Eur J Cardiothorac Surg 2009;36:84-90; discussion 90. [PubMed]
- von Oppell UO, Zilla P. Prosthetic heart valves: why biological? J Long Term Eff Med Implants 2001;11:105-13. [PubMed]
- Acar C, de Ibarra JS, Lansac E. Anterior leaflet augmentation with autologous pericardium for mitral repair in rheumatic valve insufficiency. J Heart Valve Dis 2004;13:741-6. [PubMed]
- Butany J, Collins MJ, David TE. Ruptured synthetic expanded polytetrafluoroethylene chordae tendinae. Cardiovasc Pathol 2004;13:182-4. [PubMed]
- Farivar RS, Shernan SK, Cohn LH. Late rupture of polytetrafluoroethylene neochordae after mitral valve repair. J Thorac Cardiovasc Surg 2009;137:504-6. [PubMed]
- Yau TM, El-Ghoneimi YA, Armstrong S, et al. Mitral valve repair and replacement for rheumatic disease. J Thorac Cardiovasc Surg 2000;119:53-60. [PubMed]
- Shahin GM, van der Heijden GJ, Kelder JC, et al. Long-term follow-up of mitral valve repair: a single-center experience. Med Sci Monit 2006;12:CR308-14. [PubMed]
- Talwalkar NG, Earle NR, Earle EA, et al. Mitral valve repair in patients with low left ventricular ejection fractions: early and late results. Chest 2004;126:709-15. [PubMed]
- Shahian DM, O’Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3--valve plus coronary artery bypass grafting surgery. Ann Thorac Surg 2009;88:S43-62. [PubMed]
- DiBardino DJ, ElBardissi AW, McClure RS, et al. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010;139:76-83; discussion 83-4. [PubMed]
- Rankin JS, Burrichter CA, Walton-Shirley MK, et al. Trends in mitral valve surgery: a single practice experience. J Heart Valve Dis 2009;18:359-66. [PubMed]
- Robicsek F, Payne RB, Daugherty HK, et al. Bacterial endocarditis of the mitral valve treated by excision and replacement. Ann Surg 1967;166:854-7. [PubMed]
- Dreyfus G, Serraf A, Jebara VA, et al. Valve repair in acute endocarditis. Ann Thorac Surg 1990;49:706-11; discussion 712-3. [PubMed]
- Fuzellier JF, Acar C, Jebara VA, et al. Mitral valvuloplasty during the acute phase of endocarditis. Arch Mal Coeur Vaiss 1993;86:197-201. [PubMed]
- Hendren WG, Morris AS, Rosenkranz ER, et al. Mitral valve repair for bacterial endocarditis. J Thorac Cardiovasc Surg 1992;103:124-8; discussion 128-9. [PubMed]
- Feringa HH, Shaw LJ, Poldermans D, et al. Mitral valve repair and replacement in endocarditis: a systematic review of literature. Ann Thorac Surg 2007;83:564-70. [PubMed]
- Iung B, Rousseau-Paziaud J, Cormier B, et al. Contemporary results of mitral valve repair for infective endocarditis. J Am Coll Cardiol 2004;43:386-92. [PubMed]
- Zegdi R, Debièche M, Latrémouille C, et al. Long-term results of mitral valve repair in active endocarditis. Circulation 2005;111:2532-6. [PubMed]
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006;114:e84-231. [PubMed]
- Moss RR, Humphries KH, Gao M, et al. Outcome of mitral valve repair or replacement: a comparison by propensity score analysis. Circulation 2003;108 Suppl 1:II90-7. [PubMed]
- Modi P, Rodriguez E, Hargrove WC 3rd, et al. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg 2009;137:1481-7. [PubMed]
- Woo YJ, Seeburger J, Mohr FW. Minimally invasive valve surgery. Semin Thorac Cardiovasc Surg 2007;19:289-98. [PubMed]
- Seeburger J, Falk V, Borger MA, et al. Chordae replacement versus resection for repair of isolated posterior mitral leaflet prolapse: à ègalité. Ann Thorac Surg 2009;87:1715-20. [PubMed]
- Musci M, Hübler M, Amiri A, et al. Mitral valve repair for active infective endocarditis. A 20-year, single center experience. In: Hetzer R, Rankin JS, Yankah CA. eds. Mitral Valve Repair. Springer-Verlag Berlin Heidelberg, 2011:262.
- Seeburger J, Borger MA, Doll N, et al. Comparison of outcomes of minimally invasive mitral valve surgery for posterior, anterior and bileaflet prolapse. Eur J Cardiothorac Surg 2009;36:532-8. [PubMed]
- McClure RS, Cohn LH, Wiegerinck E, et al. Early and late outcomes in minimally invasive mitral valve repair: an eleven-year experience in 707 patients. J Thorac Cardiovasc Surg 2009;137:70-5. [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. From classical sternotomy to truly endoscopic mitral valve surgery: a step by step procedure. Heart Lung Circ 2003;12:172-7. [PubMed]
- Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52. [PubMed]
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced coronary artery bypass surgery. J Thorac Cardiovasc Surg 1999;117:1212-4. [PubMed]
- Murphy DA, Miller JS, Langford DA, et al. Endoscopic robotic mitral valve surgery. J Thorac Cardiovasc Surg 2006;132:776-81. [PubMed]
- Chitwood WR Jr, Rodriguez E, Chu MW, et al. Robotic mitral valve repairs in 300 patients: a single-center experience. J Thorac Cardiovasc Surg 2008;136:436-41. [PubMed]
- Lawrie GM. Mitral valve: toward complete repairability. Surg Technol Int 2006;15:189-97. [PubMed]


