Video-assisted thoracoscopic surgery for postoperative recurrent primary spontaneous pneumothorax
Introduction
Surgical procedure, thoracotomy or video-assisted thoracoscopic surgery (VATS), can effectively reduce the recurrence rate of primary spontaneous pneumothorax (PSP) after the first episode, from 23-50% (1-3) to 3-7% (4-6). When it was first implemented for PSP, VATS seemed to have a higher postoperative recurrence rate than thoracotomy (4-6), but with gradual advancement of instrumentation and improvement of surgeon dexterity, results from both approaches are now comparable (7-9). From reports worldwide, the recurrence rate after operation for PSP is as mentioned above. Although the percentage does not seem high, any postoperative recurrence remains the embarrassment of every surgeon. Even now, there is no standard treatment principle for postoperative recurrence, and related discussions in the literature are few.
In this article, we will discuss the feasibility of re-operation and share our limited experience of implementation of Re-VATS for postoperative recurrent pneumothorax (PORP).
Materials and methods
Patients who had underwent needlescopic VATS for PSP between Jan 2007 and Dec 2011 were reviewed, with those aged 40 years and above excluded to avoid the possibility of secondary pneumothorax. Reviewed patients were followed-up once a week after discharge at our out-patient clinic for several weeks until they were satisfied with their condition. They were advised to contact us immediately in the event of any discomfort. Telephone counseling was the method of choice prior to the composition of this article.
Operative technique
Needlescopic VATS (first operation) (8).
Patients were placed under general anesthesia in the lateral decubitus position with double lumen endotracheal tube. A 10-mm camera port was inserted through the sixth or seventh intercostal space (ICS) along the mid-axillary line after lung deflation. Two 3.0-mm working ports were inserted over the 3rd ICS anterior axillary line and fifth ICS posterior axillary line, respectively. With a 10 mm 30° thoracoscopy, blebs, if identified, were grasped with an endograsper. The camera was retrieved and a 3.0 mm needlescope (Karl Storz Gmb H and Co. Tuttlingen Germany) was then inserted through the other 3-mm port for viewing. Endo GIA endoscopic linear stapler (U.S. surgical Corporation: Norwalk, CT) was then inserted through the 10 mm port for blebectomy. Any air leak and bleeding was checked. The whole parietal pleura was rubbed with marlex mesh (Bard limited, Crawly, UK). The procedure was concluded with the placing of a 28 Fr chest tube.
Diagnosis of postoperative recurrent pneumothorax (PORP)
PORP is defined as pneumothorax occurring seven days or more after removal of chest tube, all confirmed by chest computed tomography and chest radiography. The size of pneumothorax was calculated by the formula designed by Collins et al. (10). Patients diagnosed with recurrence were routinely admitted into the ward for treatment. Depending on the size of pneumothorax, treatment was either observation with O2 inhalation for pneumothorax of volume 15% or less; pleural drainage under CT guide for 16% to 30% or Re-VATS for over 30% volume.
Operative technique of Re-VATS
Patients were placed under general anesthesia in the lateral decubitus position under double lumen endotracheal intubation. A 10-mm camera port was placed through the sixth or seventh ICS or over the area of pneumothorax, avoiding the sites of old ports in case of possible pleural adhesions underneath. Two other 10-mm ports were then created under direct thoracoscopic vision, usually in the upper thorax. With ring forceps and harmonic scalpel, adhesions could be lysed if necessary. The entire lung was meticulously inspected. Blebs, if found, were resected with Endo GIA. Warm saline was then instillated to check for air leak. The pleura was then rubbed meticulously with marlex mesh. Finally, three rubber drainage tubes were placed, one at each incision, overlying different aspects of the pleural cavity. Low suction was used in the ward. The tubes were gradually retreated by 3 to 5 cm on the seventh day, and again on the ninth day. They were completely removed on the eleventh day. Patients were all followed-up in the clinic after discharge.
Results
VATS was initially performed on 239 patients with PSP in total. Eleven patients, all male, were found to have postoperative recurrence during a follow-up period of 36.95 months. Demographic data, time to postoperative recurrence, treatment, and operative data are shown in Table 1.
Three patients were treated under observation. One showed improvement and was discharged five days later. He reported no further recurrence. The remaining two recurred 12 and 9 months following, and received Re-VATS. Unfortunately, one of the two recurred again 17 days after the procedure. Since the volume was small, CT guide drainage was administered, and he was then treated with blood patch. No further recurrence was reported. Two patients received initial treatment of CT guided pleural drainage with sclerosing agent instillation afterwards. One recurred 14 days later. He then received Re-VATS and no further recurrence was reported. Six patients received Re-VATS straight off, with no further recurrence following. There was no conversion to thoracotomy, blood transfusion, prolong air leak or other complications.
Discussion
Recurrence of PSP after a first episode is a troublesome complication, and postoperative recurrence even more so. Though the latter is seemingly a minor problem in thoracic surgery, it is however, an important issue to be discussed, owing to the fact that every thoracic surgeon would face such patients during his medical practice. There is a misconception that reoperation for post-VATS recurrent pneumothorax would be quite complicated. This is because of (I) the previous pleurodesis adhesions; (II) uncertainty when looking for leakage sites. Thus many surgeons would prefer a conservative approach. Even if surgery was unavoidable, open thoracotomy would be the procedure of choice. This study found evidence of the contrary, with the authors discovered that the adhesions caused by previous abrasions in those patients with post-operative recurrence were usually mild. Some even presented with no adhesion. The reason is still unknown. (In other words, patients with fine adhesions would have no recurrence.) In this series, nine patients received Re-VATS. Only one had further recurrence of small volume, ultimately resolved with blood patch. Sixty percent (three out of five) of patients with conservative treatment finally required VATS. Only two patients receiving conservative treatment had no further recurrence indicating Re-VATS could possibly yield a more effective result.
Thoracotomy seems to be the better way to treat PORP because it allows better inspection and examination of the entire lung, and also creates greater pleural adhesions. In fact, the adhesions in the PORP would be very little for thoracoscopy as we mentioned above. In addition, inspection is much clearer with today’s high resolution video system, that even narrow hidden spaces can be carefully examined. Moreover, VATS has its benefit of better cosmetics and less pain. Usually, re-operation causes more bleeding and longer operative time. In this series, the average intra-operative bleeding was 28.33±15 mL, and operative time was 85.22±25 min (Table 1). Compared to our first VATS reported in 2009 (11), these are statistical insignificant. The hospital stay would also be longer due to deliberately lengthening the tube drainage time for pleural irritation.
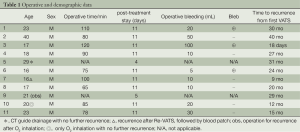
Full table
The authors do not advocate performing Re-VATS with needlescopy. The reason is the poorer resolution and narrower video-field (8,11,12). Since they are postoperative recurrent patients, a clearer video-field is essential. Moreover in our series, at the end of the procedure, rubber drains 1.0 cm in diameter, were placed in each incision, which is the reason for the three 1-cm incisions made at the start of the procedure, and a 10-mm camera is certainly superior to needlescopy.
Three patients were found to have blebs. No leakage or lesions were found for the remaining six patients. All patients received meticulous pleural abrasions, and rubber tubes over three different areas of the pleural cavity, were placed and retained for a week before slowly retreated. Rubber drains cause great tissue reaction, resulting in reliable pleural adhesions. Only one patient with leakage site not found after Re-VATS recurred, showing that good pleurodesis is a crucial part of the operation (13).
Some surgeons (7,14) perform pleurectomy during Re-VATS for greater adhesion, a step the authors in this series would not advocate, since the resulting dense adhesions would certainly be barriers to subsequent thoracotomy should the patient need it in the future. Emphasis should be placed on performing the abrasions of pleura as the last procedure of the operation after all resections and testing of leakage, as blood clots, produced by the abrasions would be an ideal sealing material, covering almost the entire lung surface, stopping even minute leakage and resulting in a satisfactory pleural symphysis.
The size of pneumothorax still guides the initial approach, however, as we can see in this series, nine out of the eleven patients received Re-VATS, showing (I) failure rate of conservative treatment is high; (II) Re-VATS could be performed with no difficulty; and (III) the results of Re-VATS were satisfactory.
Finally, we note that all of the recurrent patients were male, with five smokers among them possibly indicating that smoking is still a risk factor for pneumothorax and subsequent recurrence after surgery.
The drawback of this study is the small number of cases due to the low postoperative recurrence rate (4.6%) of PSP. Also it is a retrospective one.
In this series, all nine patients were operated on by the same surgical team. With the praising results of the present series, the authors suggest discarding the conservative treatment for PORP which many surgeons are still implementing. Even for smaller size cases, Re-VATS, which is technically feasible, safe and effective with better cosmetics and minor postoperative pain, should be a strong contender as priority treatment.
Acknowledgements
The authors thank the help of Andre Chou for article revision.
Disclosure: The authors declare no conflict of interest.
References
- Guo Y, Xie C, Rodriguez RM, et al. Factors related to recurrence of spontaneous pneumothorax. Respirology 2005;10:378-84. [PubMed]
- Ng CS, Lee TW, Wan S, et al. Video assisted thoracic surgery in the management of spontaneous pneumothorax: the current status. Postgrad Med J 2006;82:179-85. [PubMed]
- Chan SS, Lam PK. Simple aspiration as initial treatment for primary spontaneous pneumothorax: results of 91 consecutive cases. J Emerg Med 2005;28:133-8. [PubMed]
- Rena O, Massera F, Papalia E, et al. Surgical pleurodesis for Vanderschueren’s stage III primary spontaneous pneumothorax. Eur Respir J 2008;31:837-41. [PubMed]
- Chan P, Clarke P, Daniel FJ, et al. Efficacy study of video-assisted thoracoscopic surgery pleurodesis for spontaneous pneumothorax. Ann Thorac Surg 2001;71:452-4. [PubMed]
- Hatz RA, Kaps MF, Meimarakis G, et al. Long-term results after video-assisted thoracoscopic surgery for first-time and recurrent spontaneous pneumothorax. Ann Thorac Surg 2000;70:253-7. [PubMed]
- Cardillo G, Facciolo F, Giunti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg 2000;69:357-61; discussion 361-2. [PubMed]
- Chou SH, Chuang IC, Huang MF, et al. Comparison of needlescopic and conventional video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim Invasive Ther Allied Technol 2012;21:168-72. [PubMed]
- Sedrakyan A, van der Meulen J, Lewsey J, et al. Video assisted thoracic surgery for treatment of pneumothorax and lung resections: systematic review of randomised clinical trials. BMJ 2004;329:1008. [PubMed]
- Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size in chest radiographs using intrapleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol 1995;165:1127-30. [PubMed]
- Chou SH, Li HP, Lee JY, et al. Needlescopic video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim Invasive Ther Allied Technol 2009;18:221-4. [PubMed]
- Chen JS, Hsu HH, Kuo SW, et al. Needlescopic versus conventional video-assisted thoracic surgery for primary spontaneous pneumothorax: a comparative study. Ann Thorac Surg 2003;75:1080-5. [PubMed]
- Gen Thoracic Surgery. 7 ed. Chapter 58, 2009:739-61.
- Chen JS, Hsu HH, Kuo SW, et al. Management of recurrent primary spontaneous pneumothorax after thoracoscopic surgery: should observation, drainage, redo thoracoscopy, or thoracotomy be used? Surg Endosc 2009;23:2438-44. [PubMed]


