Minimally invasive surgery for atrial fibrillation
The basics
Atrial fibrillation (AF) remains the most common cardiac arrhythmia, affecting nearly 2% of the general population worldwide (1). Its prevalence increases sharply with age. A trend towards longevity, augmented by continuous advances in cardiovascular and cancer medicine will most certainly contribute to an even greater incidence of AF, especially in patients already suffering from structural heart disease. Current estimates suggest that the number of patients suffering from AF in Europe will double within the next 10 years and soar to 5 million by the year 2030. However, these numbers must be considered by taking into consideration the incidence of major adverse events associated with AF, namely stroke. It has been proven that AF is associated with an increased risk of cerebrovascular thromboembolic events, increased frequency of cardiac-related hospitalizations and a significantly reduced quality of life. The occurrence of these major adverse events raises mortality two-fold and notably increases the cost of care of patients either suffering from AF or from its non-fatal comorbidities (2-4).
Sole antiarrhythmic therapy is rarely sufficient and limited by its toxicity, and yet recent years have brought only minor improvements in this field. The highly anticipated benefits of dronedarone have doubtful efficacy (5), leaving amiodarone the drug of choice for most of the patients suffering from highly symptomatic AF. However, serious side effects such as hyperthyreosis and liver dysfunction limit its clinical potential (6). On the other hand considerable advances have been made in the field of anticoagulation, as novel oral anticoagulants (NOACs) have been widely accepted as an equally effective option to vitamin K antagonists (VKA), namely warfarin (7). This however, may be perceived as an opportunity to compensate for the ineffective methods to achieve sinus rhythm restoration, either pharmacologically or by intervention. Elimination or a significant reduction of the AF burden remains however, the most important measure of stroke prevention.
Introduction of percutaneous catheter ablation for AF in 1998 by Haissaguerre marks the foundations of modern electrophysiology. Today, endocardial catheter ablation is effective for paroxysmal AF and remains a class I/A indication in patients suffering from symptomatic recurrences despite optimal medical therapy. However, a successful treatment of persistent atrial fibrillation (PSAF) and long-standing persistent atrial fibrillation (LSPAF) remains a therapeutic challenge (8,9). Current guidelines equate surgical and catheter based ablation in terms of therapeutic efficacy (IIb) indicating that catheter ablation may be considered an effective treatment option for patients with PSAF or LSPAF (9-11). The evidence supporting this recommendation is, however, limited (C). Contrary to current guidelines, recent published data indicate the exact opposite with success rates declining over time and barely exceeding 50% at one year (12). Repeated ablations are often required, exposing the patient to extended periods of radiation and an increased risk of peri-procedural complications, and a considerably increase in the healthcare costs. On the other hand surgical ablation offers higher success rate, but its widespread acceptance is slow, mainly due to its invasive nature, having chest incisions and heart dissections. Moreover, regardless of previously published statements, European Society of Cardiology (ESC) limits surgical ablation to patients who have failed a previous catheter ablation. Given the low level of evidence (IIb/C), this recommendation remains highly controversial and rather discouraging for the large population of patients who could potentially benefit from a surgical ablation procedure to treat their PSAF or LSAF.
The key concepts
Surgical ablation as proposed by J. Cox resulted in sinus rhythm restoration in a majority of treated patients (13). His concept evolved around creation of a new pathway for the electric impulse which originates in the sinus node and ultimately ends at the atrioventricular node. By cutting and sewing certain structures of the left and right atria, Cox was able to prove his idea, subsequently calling it “the Maze” procedure. Although extremely effective even in very large atria, the Cox-Maze procedure remains technically challenging and complex, limiting its adoption in everyday clinical practice. It requires a full sternotomy, cardiopulmonary bypass and aortic cross clamping with subsequent cardiac arrest. Bleeding and complete atrioventricular block were not rare in the postoperative course of these patients. Rapid advancements in minimally invasive endoscopic and robotic cardiac surgery (14,15), allow for the re-creation of this “maze-pattern” through a minimal surgical incision (16). The introduction of the off-pump procedures, raised questions regarding lesion transmurality and efficiency of a beating heart ablation. Numerous new energy sources were introduced to facilitate this rapidly evolving field. Boundaries caused by limited access led to modifications of the original “Maze” lesion set. Yet the aim remained the same, simple and unchanged from the basic concepts:
- Isolate pulmonary veins;
- Modify substrate;
- Address left atrial appendage (LAA) whenever possible.
The aim of this review, unlike other reviews of minimally invasive surgical ablation, is to present medical professionals with two distinctly different approaches:
- Standalone surgical isolation of the pulmonary veins using bipolar energy source with concomitant amputation of the LAA;
- The Convergent Procedure, a multidisciplinary approach that combines both endocardial and epicardial unipolar ablation.
Standalone surgical ablation
Isolation of the pulmonary veins as right and left pairs through bilateral mini-thoracotomies performed off-pump using a bipolar radiofrequency device was first introduced by Randal Wolf in 2005 (17). The “Wolf procedure” included bilateral antrum isolation (similar in concept of Pappone) and partial cardiac denervation and resulted in impressive outcomes. Yet, it required multiple chest incisions and lung deflation. A quick march towards totally endoscopic ablation led to oversimplified concept of a “box” lesion-a single circular lesion which encompassed all pulmonary veins together with the posterior wall of the left atrium. The “Box lesion” was made possible (or was it created…?) by rapid development of ablating devices, utilizing either microwave (early years, abandoned) or unipolar radiofrequency (still used today) energy to create circumferential thermal injury to the left atrial wall. Since the construction of these devices was fairly simple and constituted of a single tube with heating element located on the inner side of the tube, its introduction into the pericardial sac, and around the LA remained quite humble and required minimal endoscopic skills (18,19). However breaking through pericardial reflections, especially one separating superior vena cava from the right pulmonary artery, was cumbersome and not once resulted in both SVC and LA laceration and bleeding. Introduction of various type of add-ons facilitating probe delivery and retrieval (magnets) made this quick and widely accepted surgical procedure. However, it proved to be moderately successful in SR restoration. More importantly, it addressed the need for treatment options for patients with paroxysmal and persistent who at that time had been receiving treatment that was less invasive and at least as effective with percutaneous techniques. The concept of a “box” lesion evolved with several modifications, one especially worth mentioning. In 2011 Munaretto introduced a semi hybrid approach using a RF device (Estech, Cobra Adhere XL) to create a primary “box” lesion, similar to the classic lesion pattern (20). Other modifications followed such as additional three lesions were placed on the lateral wall of the right atrium, creating a triangular area of isolated myocardium. The so called “Brescia Lesion Set” was associated with much higher success rate (over 85%) than the box alone (nearly 60% in SR at one year). The procedure was then enhanced by percutaneous catheter based endocardial ablation, but only in patients who had recurrences after the initial treatment. Should it be considered as the true hybrid or bail out remains an open question. The important message is, that such an approach yields an impressive, near 90% success rate, measured with the implantable loop recorders (21).
On the contrary, Wolf’s procedure utilizing bipolar energy, was reintroduced with video assisted techniques, and evolved into the most complex, totally endoscopic ablation procedure to date, targeting not only pulmonary veins, but adding supporting lesions to the mitral or aortic trigones, addressing the ligament of Marshall, ganglionated plexi and LAA all at the same time (22). Although various devices appeared on the market, one manufactured by AtriCure gained attention, as it was the system of choice used in the surgical arm of the Atrial Fibrillation Catheter Ablation Versus Surgical Ablation Treatment (FAST) Trial. The FAST Trial was the first prospective randomized clinical trial was designed to compare catheter and surgical ablation in a well-described population of patients with AF (23). It randomized one hundred twenty-four patients into two treatment arms. The results were unfavorable for catheter based procedures, as one year post procedure only 36% of patient in the percutaneous group were free form AF and AADs compared with 65% treated surgically. Even with AADs freedom from AF remained significantly higher in surgical group (78% vs. 42%). Of noteworthy is however, that as many as 23% of patient in the FAST trial were diagnosed with paroxysmal AF and 55% presented in sinus rhythm at admission. This rare comparison showed however, that surgical ablation was superior to catheter ablation in achieving freedom from left atrial arrhythmias after 12 months of follow-up, although the procedural adverse event rate was significantly higher for the surgical group. Most of these were minor and included pneumothorax (6 cases!), a rib fracture and one conversion to sternotomy. Sadly, it was not the success rate, but the number of periprocedural complications that was most remembered by the general public. Therefore it is crucial for minimally invasive techniques to remain truly minimal in all the aspects of care. Unfortunately, the FAST trial remains the only randomized clinical trial directly comparing surgical versus percutaneous treatment options.
The standalone surgical ablation procedure
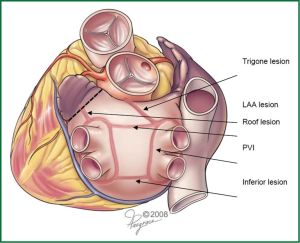
The totally thoracoscopic, off-pump, bilateral extended ablation with the ganglionated plexi (GP) ablation and the LAA amputation procedure is based on the bipolar radiofrequency technology, which has long-established data on excellent transmurality. The lesion pattern involves pulmonary vein isolation in pairs, isolation of the posterior aspect of the LA and the “triagonal” lesion extending from the box to the non-coronary aortic annulus. This line is similar in its purpose to the left mitral isthmus line in the classical MAZE concept (Figure 1).
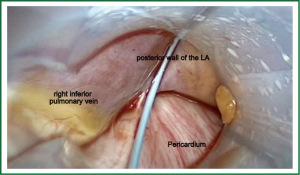
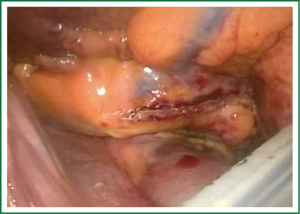
Patient is placed in the supine position under general anesthesia and intubated using double lumen intratracheal tube to facilitate single lung ventilation. Three thoracoscopic ports are placed on both sides of the chest through the 4th (camera port) and 6th (working ports) intercostal spaces in the midaxillary line. First the right chest is entered, then the pericardium is opened above the phrenic nerve, exposing the right pulmonary veins, together with Waterstone’s Groove. The identification of GP followed by a high-frequency (1,000 hz, 18 V) pacing-induced vagal response, which is defined by a ventricular asystole of at least 3 seconds or ventricular rate slowing of more than 50% when AF is present. Once identified, GPs are then ablated using an unipolar pen-like device (Figure 2). Subsequent pacing and continuation of the procedure further along the Waterson’s groove serve as the confirmation of the performed applications. Next, the oblique and transverse sinuses are bluntly opened and the AtriCure Lumitip Dissector (AtriCure, Inc., West Chester, Ohio, USA) is introduced around both upper and lower pulmonary veins. At least three overlapping ablation lesions are performed at the antrum of the veins (8). Bidirectional acute conduction block is confirmed both by absence of sensed atrial potentials in the PVs and pacing of the PVs in patients in sinus rhythm. An additional ablation lesion is created if necessary. Subsequently additional lesions using the bipolar linear device are performed to close the box pattern lesion and so-called triagonal line between the roof of the left atrium and the non-coronary aortic annulus. Then the left thoracic cavity is entered in a similar fashion. Left pericardium is opened posterior to the phrenic nerve and both upper and lower pulmonary veins are encircled with the bipolar clamp. Again, minimum of 3 overlapping applications are performed. Dissection of the ligament of Marshall using electrocautery and amputation of the LAA are performed last. LAA is usually addressed with a GI stapler and entirely removed. Extreme caution must be taken during this part of the procedure, as LAA may be torn easily. Moreover a stapler must be well positioned, not to leave any remnants, as these may be even stronger triggers that predispose thrombus formation.
Recent modification of the abovementioned technique lead to multidisciplinary procedure, with electrophysiology specialists performing either right-sided lesions or completing left sided, trigonal or isthmus lines. Initial results of this combined approach are very encouraging, especially in patients with LSPAF (24,25).
The convergent approach
The concept of the combined surgical (epicardial ablation) and electrophysiological (endocardial ablation) approach to treat AF was introduced in 2009, and has evolved over time to what is now referred to as the Convergent Procedure-a collaborative effort of cardiac surgeons and electrophysiologists. Over time the Convergent Procedure has been adopted by increasing number of centers as the positive outcomes demonstrated by the early adopting centers were published (26-28). Now, the term “Convergent Approach/Procedure” is widely used, to define the joint efforts of electropysiologists and cardiac surgeons who perform the epicardial and endocardial ablation as a multidisciplinary Convergent approach for the treatment of AF.
The convergent procedure
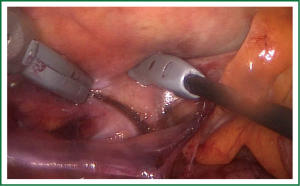
The Convergent Procedure is a collaborative effort of both the cardiac surgeon and the electrophysiologist. The patient is selected and scheduled for the Convergent Procedure after both the cardiac surgeon and the electrophysiologist has completed their exam and evaluation of the identified patient. Pre procedural procedures are followed per physician and facility standard of care. A TEE is performed to identify presence/absence of any left atrial clots. After the patient is anesthetized trans-diaphragmatic access is obtained using standard laparoscopic surgical techniques for creating a pericardial window, to access the posterior surface of the heart. A 3 cm incision is created below the xyphoid in the midline of the abdomen. Using CO2 insufflation an incision is created through the central tendon of the diaphragm, above the liver and medial to the falciform ligament. The cannula (nContact Surgical Inc., Morrisville NC, USA) is inserted through the incision to access the posterior surface of the heart. The cannula provides the conduit to insert the scopes and the Numeris® or EPi-SenseTM Guided Coagulation System with VisTrax® (nContact Surgical Inc., Morrisville NC, USA). The Numeris or the EPi-Sense device is used to create the epicardial lesions (Figure 3). By gentle manipulation of the endoscope, the surgeon is able to clearly visualize the posterior aspect of the left atrium and both right and left pulmonary veins irrespective of their anatomical variations (Figure 4). The electrode–designed to fit within the narrow working channel created by the cannula, is then placed inside the pericardium, oriented towards the structures to be ablated. The coagulation device utilizes vacuum to create consistent contact between the 3 cm unipolar radiofrequency electrode and epicardial tissue. The vacuum additionally pulls saline through the device to cool the surface not intended for ablation thereby directing energy only into epicardial tissue pulled into engagement with the ablation electrode. The Radiofrequency Generator utilizes an algorithm based on impedance that regulates power to prevent tissue overheating and subsequent vaporization. Using a step-by-step approach, a comprehensive ablation pattern is then created. Posterior wall of the left atrium is ablated first, then posterior surfaces of right and left pulmonary veins. Then the cannula is moved towards the right to visualize and ablate anterior aspect of the left pulmonary veins. In some cases visualization and subsequent ablation of the ligament of Marshall is also completed. Lastly, anterior surface of the right pulmonary veins is ablated, together with fat overlying the Waterson’s Groove—a known site of a multitude of GP.
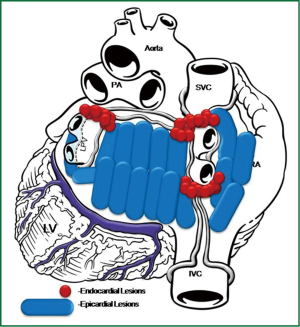
All RF applications on the posterior wall of the left atrium are performed under fluoroscopic guidance to visualize the relation between the ablating electrode and the esophagus. A temperature probe, placed prior to the skin incision in the esophagus is necessary, as it is moved to match the position of the ablating electrode. This allows safe ablation of atrial structures located in close proximity or just anterior to the esophagus. A saline flush just prior to RF energy application submerges the ablating electrode and cools the device and the surrounding tissue. When all lesions are created, a small drain is placed behind the LA and passed through one of the 5 mm endoscopic ports. The midline fascia is closed with interrupted permanent sutures and skin and port incisions are closed using absorbable sutures. While transdiaphragmatic access offers an excellent opportunity to address the posterior wall of the left atrium and create a large area of non-viable scar tissue to modify the substrate, it does not allow for complete circumvention of either right or left pulmonary veins as the pericardial reflections, located just superior to right and left upper pulmonary veins and a ridge connecting right inferior pulmonary vein with inferior vena cava, are not dissected. The second step–endocardial ablation, is performed to complete the isolation of the PVIs. The Brockenbrough needle and the Mullins-type transseptal sheath are both positioned in the upper right atrium and the transseptal puncture is performed under the guidance of intracardiac pressures recorded from the tip of the needle. With the use of an electro-anatomical mapping system the isopotential map of the left atrium is then created to identify areas, showing electrical activity within the pulmonary vein ostia and in the region of left atrial isthmus (Figure 5). Once identified and marked on the map, areas with persistent conductivity are ablated using radiofrequency applications. Finally, the electrical isolation of all structures is verified by rapid pacing. This concludes the convergent procedure, and the patient is usually extubated on the operating table and discharged home on postoperative day 2-4.
Worldwide adoption and therapeutic success
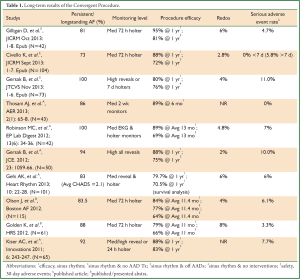
The transdiaphragmantic, Convergent Procedure for the treatment of AF has been successfully implemented in over 60 centers around the globe. With over 2,000 procedures performed worldwide. The Convergent Procedure has become the most frequently performed multidisciplinary procedure for a minimally invasively treatment of AF. Data published provide compelling evidence supporting the efficacy and safety of this combined approach (29). While most of this evidence comes from single-center, non-randomized clinical studies, the centers treat a similar patient population and report comparable early and long-term results (Table 1). This is especially important, when peri-procedural differences are taken into consideration. The two key procedures—endocardial and epicardial, or surgical and endocardial, remain unchanged in their design and performance. It is the sequence, or timing between the two procedures, that may vary due to country specific reimbursement issues. In the United States the Convergent Procedure is truly a convergent procedure, as the two steps epicardial and endocardial ablations are performed sequentially during the same procedure. On the other hand, some European countries stage the epicardial and endocardial ablation procedures 7 to 21 days apart, accomodating the country’s reimbursement policy requirements. Although it is better for hospital economics, the dual stage convergent procedure is less satisfactory for the patient as a second hospitalization and a second TEE prior to the endocardial ablation are needed. Prior to the procedure, patients are informed about the nature of the staged procedure and seem to understand and accept its limitations. However, some patients may remain or revert to AF and some patients may be reluctant to undergo the endocardial ablation because of rhythm restoration and rapid improvement in quality of life. The one big benefit of the staged procedure is the clear delineation of periprocedural complications, either to the surgical or EP procedure. To mitigate the risk of thromboembolic events post procedural anticoagulation is of the utmost importance. Low molecular weight heparin (LMWH) may be used as a bridging anticoagulant and patients should be started on anticoagulant therapy regimen immediately after the procedure. Novel OACs have proved effective and safe in this population, although larger studies are needed to fully evaluate their role in post ablation procedure settings.
Full Table
Procedural efficacy reported by centers, range from 79% to 95% of treated patients in stable sinus rhythm one-year post procedure. Differences in reported outcomes from various centers may be due to various factors such as operators experience, overall patient volume, baseline characteristics, and most importantly, due to differences in rhythm monitoring tools used to assess recurrence of AF. Medical practice recommends longer period monitoring, such as a 7 day holter, on the other hand the Heart Rhythm Society and European Guidelines recommend minimally a 24 hour holter for efficacy evaluation for clinical studies. A few centers have adopted the use of continuous implantable loop recorders (Medtronic Reveal XT), however, this remains a costly option and is not reimbursed in most countries. The implantable loop recorders provide continuous monitoring of the patient’s rhythm and a relative accurate measurement of procedural effectiveness. The rate of periprocedural complications also differs among centers from none, to 11%. Mild pericarditis remains the most frequently encountered early complication, associated with extensive thermal trauma, which resolves quickly with pharmacological treatment. Stroke and transient ischemic attack (TIA), were reported in some publications as post procedural complications that occurred within the first 30 days. Esophageal fistulas were reported by one or two centers, and occurred during the first years of technical development. Since 2010, this complication has not been encountered. Bleeding, leading to tamponade and requiring immediate surgical assistance was also reported. It remains an infrequent complication of all minimally invasive cardiothoracic procedures (30). This risk is mitigated in the Convergent Procedure due to the placement of a chest tube drain after the epicardial ablation portion of the procedure.
The concept of a combined approach, brings together the most effective techniques of both surgical and endocardial catheter ablation resulting in the creation of the Convergent Procedure. This novel pericardioscopic, Convergent approach is demonstrating success as an effective option for symptomatic patients with PSAF and LSPAF for whom the standalone surgical or endocardial ablation procedures offer low single procedure success rates.
LAA exclusion
LAA, may also be addressed when convergent ablation is performed. Although LAA may be sufficiently visualized with pericardioscopy, its exclusion using this tactic is impossible, mainly due to limited working space within tubular cannula. However, at our center we have adopted minimally invasive thoracoscopic techniques to successfully eliminate the LAA during this procedure. By entering the left thoracic space with an endoscope, the LAA is removed using a GI stapling device (Figure 6). Then, the same ablating electrode can be used to ablate the anterior and superior aspects of the left pulmonary veins, together with the ligament of Marshall. Such an access also allows for placement of the ablating electrode at the roof of the left atrium under direct visibility. Once the LAA exclusion procedure is performed, the ports are removed from the chest, dual ventilation is commenced and the transdiaphragmatic ablation of structures not previously ablated follows. Although our center has not experienced any safety issues this should only be reserved for patients in whom chronic anticoagulation is contraindicated or a history of stroke or TIA despite oral anticoagulation is documented.
The other options are to perform percutaneous closure of LAA using either Watchman (endocardial) or Lariat (epicardial) devices, either during surgical (Lariat) or endocardial (Watchman) portion or stage of the ablation.
Final remarks
Minimally Invasive surgical ablation of AF remains one of the most dynamically evolving fields of modern cardiac surgery. While there are more than a dozen issues driving this development, two seem to play the most important role: First–there is lack of evidence supporting percutaneous catheter based approach to treat patients with persistent and long-standing persistent AF. Paucity of this data offers surgical community unparalleled opportunity to challenge guidelines and change indications for surgical intervention. Large, multicenter prospective clinical studies, similar to the FAST are therefore of utmost importance, as well as honest, clear data reporting. Second—a collaborative methodology started a long-awaited debate on a Heart Team approach to AF, similar to the debate on coronary artery disease and transcatheter valves. Appropriate patient selection and tailored treatment options will most certainly result in better outcomes and patient satisfaction, coupled with appropriate use of always-limited institutional resources.
Acknowledgements
Disclosure: the authors declare no conflict of interest.
References
- Romero JR, Wolf PA. Epidemiology of Stroke: Legacy of the Framingham Heart Study. Glob Heart 2013;8:67-75. [PubMed]
- Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation 2007;115:3050-6. [PubMed]
- Chugh SS, Blackshear JL, Shen WK, et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol 2001;37:371-8. [PubMed]
- Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. [PubMed]
- Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011;365:2268-76. [PubMed]
- Vorperian VR, Havighurst TC, Miller S, et al. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol 1997;30:791-8. [PubMed]
- Sairaku A, Yoshida Y, Ando M, et al. A head-to-head comparison of periprocedural coagulability under anticoagulation with rivaroxaban versus dabigatran in patients undergoing ablation of atrial fibrillation. Clin Drug Investig 2013;33:847-53. [PubMed]
- Ad N, Henry L, Hunt S. The impact of surgical ablation in patients with low ejection fraction, heart failure, and atrial fibrillation. Eur J Cardiothorac Surg 2011;40:70-6. [PubMed]
- Letsas KP, Efremidis M, Charalampous C, et al. Current ablation strategies for persistent and long-standing persistent atrial fibrillation. Cardiol Res Pract 2011;2011:376969.
- European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. [PubMed]
- Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:e257-354. [PubMed]
- Oral H, Chugh A, Good E, et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 2007;115:2606-12. [PubMed]
- Cox JL, Boineau JP, Schuessler RB, et al. Electrophysiologic basis, surgical development, and clinical results of the maze procedure for atrial flutter and atrial fibrillation. Adv Card Surg 1995;6:1-67. [PubMed]
- Suwalski P, Suwalski G, Wilimski R, et al. Minimally invasive off-pump video-assisted endoscopic surgical pulmonary vein isolation using bipolar radiofrequency ablation - preliminary report. Kardiol Pol 2007;65:370-4; discussion 375-6. [PubMed]
- Gerosa G, Bianco R, Buja G, et al. Totally endoscopic robotic-guided pulmonary veins ablation: an alternative method for the treatment of atrial fibrillation. Eur J Cardiothorac Surg 2004;26:450-2. [PubMed]
- Kiser AC, Wimmer-Greinecker G, Chitwood WR. Totally extracardiac Maze procedure performed on the beating heart. Ann Thorac Surg 2007;84:1783-5. [PubMed]
- Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:797-802. [PubMed]
- Saltman AE, Rosenthal LS, Francalancia NA, et al. A completely endoscopic approach to microwave ablation for atrial fibrillation. Heart Surg Forum 2003;6:E38-41. [PubMed]
- Bisleri G, Bottio T, Manzato A, et al. Surgical treatment of lone atrial fibrillation in an awake patient. Heart Surg Forum 2005;8:E158-60. [PubMed]
- Muneretto C, Bisleri G, Bontempi L, et al. Successful treatment of lone persistent atrial fibrillation by means of a hybrid thoracoscopic-transcatheter approach. Innovations (Phila) 2012;7:254-8. [PubMed]
- Muneretto C, Bisleri G, Bontempi L, et al. Durable staged hybrid ablation with thoracoscopic and percutaneous approach for treatment of long-standing atrial fibrillation: a 30-month assessment with continuous monitoring. J Thorac Cardiovasc Surg 2012;144:1460-5; discussion 1465. [PubMed]
- Edgerton JR, Jackman WM, Mack MJ. A new epicardial lesion set for minimal access left atrial maze: the Dallas lesion set. Ann Thorac Surg 2009;88:1655-7. [PubMed]
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [PubMed]
- La Meir M, Gelsomino S, Lucà F, et al. Minimally invasive thoracoscopic hybrid treatment of lone atrial fibrillation: early results of monopolar versus bipolar radiofrequency source. Interact Cardiovasc Thorac Surg 2012;14:445-50. [PubMed]
- Weimar T, Vosseler M, Czesla M, et al. Approaching a paradigm shift: endoscopic ablation of lone atrial fibrillation on the beating heart. Ann Thorac Surg 2012;94:1886-92. [PubMed]
- Zembala M, Filipiak K, Kowalski O, et al. Minimally invasive hybrid ablation procedure for the treatment of persistent atrial fibrillation: one year results. Kardiol Pol 2012;70:819-28. [PubMed]
- Gersak B, Pernat A, Robic B, et al. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:1059-66. [PubMed]
- Kiser AC, Landers M, Horton R, et al. The convergent procedure: a multidisciplinary atrial fibrillation treatment. Heart Surg Forum 2010;13:E317-21. [PubMed]
- Geršak B, Zembala MO, Müller D, et al. European experience of the convergent atrial fibrillation procedure: Multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg 2013. [Epub ahead of print]. [PubMed]
- Salenger R, Lahey SJ, Saltman AE. The completely endoscopic treatment of atrial fibrillation: report on the first 14 patients with early results. Heart Surg Forum 2004;7:E555-8. [PubMed]