VATS lobectomy facilitates the delivery of adjuvant docetaxel-carboplatin chemotherapy in patients with non-small cell lung cancer
Introduction
With an ever-increasing array of novel therapeutics competing for clinical resources, the rate at which new cancer treatments can be evaluated and made available to under-served populations depends heavily on the recruitment of patients into mid-stage and late-stage clinical trials. For many cancers, including non-small cell lung cancer (NSCLC), this rate of development traditionally has been slow; many trials require years to complete patient accrual, while others close enrollment prior to attaining target sample sizes and compromise the studies’ power to fully evaluate their therapeutic strategies (1). This problem has been particularly pronounced in the area of post-operative adjuvant therapies. The China Clinical Trials Consortium (CCTC), a cooperative group formed to facilitate the development of advanced clinical research in China, has been designed to access in a rapid, efficient and rigorous manner extremely large pools of cancer patients previously unavailable to participate in the development of new cancer therapies.
Compared to many other cancers, overall survival among patients with NSCLC remains poor, even when the disease is discovered in its earliest stages (2). Attempt at curative resection even for stage IA patients, for example, is associated with only 70-75% survival (3,4); survival after resection for stages II-IIIA ranges from 30-50% (4). Early, undetected metastasis is responsible for the vast majority of the failures of these local treatments, and adjuvant systemic therapies for NSCLC have therefore been investigated extensively. Whereas alkylating agents do not seem to add any survival benefit in this patient population, platinum-based combination (doublet) therapies have consistently been shown to confer an absolute 5-year survival benefit ranging between 4% and 15% (5-9).
Positive randomized studies of adjuvant chemotherapy for NSCLC have studied combinations of cisplatin with either etoposide or a vinca alkaloid (e.g., vinorelbine), or of carboplatin and paclitaxel (6-9). The combination of carboplatin and docetaxel has been studied in patients with advanced-stage NSCLC in patients who had not received any prior therapy (10). In that study, the efficacy of this combination was comparable to that of a cisplatin-vinorelbine doublet. Grade 3 or 4 toxicity in the form of nausea, vomiting or anemia, however, was higher among patients receiving vinorelbine than among patients receiving docetaxel combined either with cisplatin or carboplatin. A number of quality of life parameters were also improved in the docetaxel arms compared to the patients who had received vinorelbine.
Benefit from chemotherapy, whether administered as a first line agent for late-stage patients or in the adjuvant setting after an attempt at curative resection, depends on successful completion of a prescribed course of therapy (11). In this regard, successful completion of adjuvant regimens has been somewhat disappointing in previously reported studies (7,8), likely related to the reduced functional status of patients still recovering from anatomic lung resection. In two studies of adjuvant cisplatin-vinorelbine, for example, only 58-61% of eligible patients completed at least 3 cycles of post-operative chemotherapy; in one study 77% of patients required at least one dose reduction or omission (7), while in the other 62% of patients received <66% of the planned dose of vinorelbine and 37% tolerated only <66% of the total dose of cisplatin (8). The combination of docetaxel and carboplatin, on the other hand, has been well-tolerated as a first line therapy for late stage patients (10). Only one study, however, has evaluated the safety and tolerability of this regimen in the post-operative setting, and its small sample size and long accrual period likely compromised its ability to establish its pre-defined safety target (12). We therefore assessed these parameters in a cohort of 133 patients in an open-label, single arm study. The CCTC was enlisted to accelerate completion of the study.
Methods
Study Design and Patients
For this open label study, the primary endpoint of safety was defined as a febrile neutropenia rate of 10% or less. A Simon’s two-stage sequential design (13) indicated that a sample size of 133 would yield a power of 90% with the alpha set 5% to test the null hypothesis that P<0.850 versus the alternative hypothesis that P≥0.930 where P was the absence of febrile neutropenia. Accordingly, the combination of docetaxel and carboplatin would be rejected if 14 or more cases of febrile neutropenia were observed in conjunction with this adjuvant therapy.
Enrollment of patients took place at a single center in the United States (Dartmouth-Hitchcock Norris Cotton Cancer Center) and at ten sites in China that were early members of the CCTC. Five patients were enrolled during a pilot phase at CCTC sites between July and September 2009. The remaining 128 patients were subsequently enrolled within a six-month period between October 2009 and April 2010. Two patients were enrolled during this period in the US; 39, 45 and 47 patients were enrolled at centers in Beijing-Tianjin, Guangzhou and Shanghai, respectively. The protocol was approved by an ethics committee at each center, and was conducted according to the Declaration of Helsinki and the International Committee on Harmonization guidelines for Good Clinical Practice (ICH-GCP, E6). Every patient provided written informed consent according to GCP guidelines. An independent data and safety monitoring board was also appointed.
Eligibility criteria included: 2-8 weeks within complete resection (R0) of pathologically confirmed NSCLC, stage IB-IIIA according to IASLC 7th TNM staging system (14), via lobectomy, bilobectomy or pneumonectomy plus formal lymph node dissection; age >17 and ECOG status 0-1 with normal organ function based on blood counts and chemistries. Patients with concurrent malignancies or who received any prior therapy for NSCLC were excluded, as were HIV-positive patients and patients with grade 2 or higher neuropathy, treatment within 30 days with any other investigational anti-cancer agent, previous treatment with docetaxel or carboplatin, or hypersensitivity to platinum.
Procedures
Patients were registered in the study after undergoing resection with curative intent and after confirmation of eligibility criteria. After providing written informed consent, patients were prescribed a total of three courses of combination therapy with docetaxel (75 mg/m2 IV) and carboplatin [AUC 5.5 × (estimated creatinine +25)]. Docetaxel was administered before carboplatin on the first day of each three-week cycle. Complete blood counts were measured each week until the completion of all three cycles, and blood chemistries were measured prior to initiation of each cycle. An absolute neutrophil count of at least 1,200/mm3 was required before each cycle, and specified dose modifications were instituted for various grades of neutropenia and/or thrombocytopenia. Patients requiring more than 2 dose reductions were removed from the study; colony-stimulating factors were used at the discretion of physicians according to published American Society of Clinical Oncology (ASCO) guidelines. Patient follow up visits were scheduled at 3, 6, 12, 18 and 24 months after the last dose of chemotherapy, and every 12 months thereafter until the 60-month follow up is reached.
Statistical analysis
The primary endpoint was defined as the rate of febrile neutropenia associated with the adjuvant administration of docetaxel/carboplatin. Secondary endpoints such as rates of adverse events (AEs) and serious adverse events (SAEs), specific toxicities (based on highest grade according to Common Terminology Criteria for Adverse Events, version 4.0), dose modifications, removal from the study, and completion of the three prescribed cycles of chemotherapy were assessed as indicators of the regimen’s tolerability. Post-hoc comparison of chemotherapy utilization was made between patients undergoing a minimally invasive video-assisted thoracoscopic surgery (VATS) approach versus traditional open thoracotomy. Dichotomous variables were compared using a Chi-squared test, while continuous variables were compared using Student’s t-test. A P-value of <0.05 was considered statistically significant.
Results
After a preliminary three-month period of protocol initiation, 96% of patient enrollment was completed between October 2009 and April 2010. A total of 89 men (67%) and 44 women (33%) were enrolled; patient characteristics are summarized in Table 1. Lobectomy was performed in 118 patients (89%, including 3 sleeve lobectomies), while the remaining patients received either bilobectomy (7.5%) or pneumonectomy (8.6%). Sixty-six procedures (50%) were performed using a VATS approach, including 62 lobectomies (53% of lobectomies), 1 bilobectomy (14% of bilobectomies), and 3 pneumonectomies (38% of pneumonectomies).

Full Table
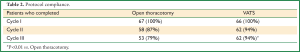
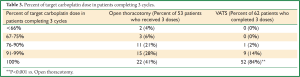
A total of 115 patients (86%) completed three cycles of docetaxel-carboplatin therapy according to protocol (Table 2). Discontinuation of therapy resulted from adverse reactions in 6 cases (4.5%) and patient withdrawal in 12 (9%). Among patients receiving all three cycles, only 2 (1.5%) received less than 66% of their target doses of carboplatin, and 3 (2.3%) received between 67 and 75% of their carboplatin doses, all 5 tolerating their full docetaxel doses (Table 3). Docetaxel dose reduction was required in only 4 patients (3.5%) receiving all three cycles. Among patients receiving all three cycles, 74 (64%) received full dose chemotherapy; 56% of patients in the study therefore received all three cycles of full-dose chemotherapy. Utilization of a VATS approach for resection was associated with a lower rate of therapy discontinuation; 62 of 66 VATS patients compared to 53 of 67 open thoracotomy patients received all three doses according to protocol (P<0.01). In addition, only 1 VATS patient receiving all three doses required a dose reduction of >10% of the target doses, compared to 16 patients undergoing open thoracotomy (P<0.001). Interestingly, there was no difference in the time from surgery to initiation of chemotherapy between the VATS patients and the open thoracotomy patients (32±10 and 34±9 days, respectively, P=0.4).
Full Table
Full Table
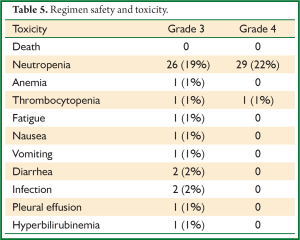
Febrile neutropenia was encountered in a total of 12 patients (9.0%), below the pre-defined safety threshold rate of 14 cases. Five cases occurred after the second cycle of adjuvant chemotherapy, with the remainder occurring after the third cycle. Hospitalization for febrile neutropenia was required only in 1 case; each case resolved without serious complication (Table 4). Four VATS patients and 8 open thoracotomy patients experienced febrile neutropenia (P=0.26). There were no treatment related deaths, and grade 3 or 4 toxicities are reported in Table 5. Some degree of neutropenia was observed in 74 patients (56%); grade 3 or 4 neutropenia was observed in 55 patients (41%). Grade 3 or 4 nausea, vomiting or diarrhea was encountered in only 3 patients (2%) during the course of therapy.

Full Table
Full Table
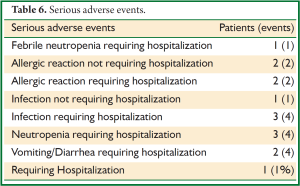
Eighteen serious adverse events (SAEs) were encountered in 14 (10.5%) patients, including 4 allergic reactions to docetaxel that required discontinuation of therapy, two of which required hospitalization for hypotension (Table 6). Nine of the other SAEs involved hospitalization, either for febrile neutropenia (1 patient), infection (3 patients), severe neutropenia (3 patients) or severe vomiting/diarrhea (2 patients); the last SAE involved a pelvic infection requiring intravenous antibiotics.
Full Table
Discussion
According to the pre-specified criteria, the combination of docetaxel and carboplatin was determined in this study to be safe in the adjuvant treatment of NSCLC. There were no treatment-related deaths. Febrile neutropenia was encountered in 9.0% of patients, which is comparable to febrile neutropenia rates of 5-14% reported with other common regimens for NSCLC, either in the first line or adjuvant setting (5-10,15). The rate of treatment-related hospitalization (11/133, 8.3%) was similarly low, with the only other SAEs involving allergic reactions or infection that did not require hospitalization. Other Grade 3 or 4 toxicities were also quite comparable in incidence to the reported literature for other first line and adjuvant therapies for NSCLC (5-10,15).
Accrual of patients in this study occurred at an unusually rapid pace compared to other recent trials involving adjuvant therapy after resection of NSCLC with curative intent (6-10). In fact, several important adjuvant trials have been halted due to poor patient accrual (6,16). This efficient enrollment was accomplished via the participation of the CCTC, an organization recently formed to advance fully ICH-GCP compliant clinical research at leading cancer centers in China. Although concern was raised prior to study initiation about the use of ‘Western’ doses of chemotherapy in an Asian population that often receives reduced doses (17), the full dose of docetaxel and carboplatin was well-tolerated in this study. In fact, as has been seen in a previous study of carboplatin-based adjuvant chemotherapy for NSCLC (9), compliance with the treatment protocol was high, with 86% of patients receiving all three cycles, compared to approximately 60% of patients in adjuvant studies of vinorelbine and cisplatin (7,8). Enforcement of strict protocol adherence among CCTC investigators and the tolerability of the regimen under study were likely responsible for the high compliance rate observed.
A similar study of the feasibility of docetaxel and carboplatin in the adjuvant treatment of NSCLC was recently conducted at seven centers in the United States (12). Although that study failed to meet its predetermined safety and feasibility target by a very small margin, the overall results were very similar to the current study. Those investigators reported completion of the study regimen in 79% of patients, and a febrile neutropenia rate of 11%. Interestingly, however, nearly two and a half years were required to complete enrollment of 72 patients in that phase II trial. Had those investigators attempted to accrue the sample size of 133 patients included in this study, enrollment at the same pace would have required four and a half years, compared to the enrollment period of approximately 6 months in this CCTC clinical trial.
In this initial study, the CCTC was therefore able to accelerate enrollment by nearly an order of magnitude over accrual in a comparable American trial. Importantly, this accrual speed did not result in a compromise of protocol or GCP compliance. Similar acceleration of other important, large-scale clinical projects may transform current capabilities for the development of novel cancer therapies. An unprecedented ability to rapidly conduct several phase II studies of a single agent in parallel, for example, may allow a much broader range of indications for a novel therapeutic to receive reasonable consideration, and may prevent the oversight of an important new indication for drug usage that might have resulted from a previous limitation of clinical resources.
This study was not designed prospectively to compare a traditional open thoracotomy to a more minimally invasive VATS approach to anatomic resection, and patients were therefore not randomized between these two forms of surgery. A relatively comparable of patients in our cohort, however, received each form of treatment, which allowed some post hoc comparisons to be made. In another retrospective, non-randomized study that compared 43 patients who underwent complete resection by thoracotomy to 57 patients treated via thoracoscopy (18), VATS lobectomy patients had statistically significantly fewer delayed doses and fewer dose reductions than thoracotomy patients. In addition, 61% of VATS patients versus 40% of thoracotomy patients in that study received 75% or more of their planned adjuvant regimen (P=0.03). In comparison, only 57% of the thoracotomy patients in the Cancer and Leukemia Group B trial 9633 received full-dose chemotherapy (19), and the Intergroup JBR.10 trial reported that 55% of their thoracotomy patients had at least 1 dose delay (7). Similarly, only 34% of patients in the chemotherapy arm of the Adjuvant Lung Project Italy series received all scheduled doses without adjustment or delay; only 69% completed their treatments with or without adjustments or delay (20). The potential benefit of a minimally invasive surgical approach, with a likely reduction in post-operative pain and an easier post-operative recovery period prior to initiation of chemotherapy (21), was supported by the observation that treatment protocol compliance and delivery of target doses of adjuvant chemotherapy were higher among patients undergoing VATS lobectomy in the current study.
Taken together, the results of this study suggest that the combination of docetaxel and carboplatin is both safe and well-tolerated in the adjuvant treatment of NSCLC, and that adjuvant treatment compliance was highest among patients undergoing a minimally invasive surgical approach. Ongoing follow-up of the cohort of patients enrolled in this study will provide some insight into the relative efficacy of this regimen compared to the reported experience in other adjuvant studies. Perhaps more important, this study also demonstrated successful implementation of the founding premise of the CCTC: that substantial improvement in the current landscape for the development of novel cancer therapies can be achieved through rigorous organization and management of dedicated clinical study groups involving emerging centers of excellence in cancer care and research.
Acknowledgement
This study was supported by a grant from Sanofi-Aventis.
Disclosure: No potential conflict of interest. Presented at the 14th World Conference on Lung Cancer, Amsterdam, July 6, 2011.
References
- Zhao Y, Kosorok MR, Zeng D. Reinforcement learning design for cancer clinical trials. Stat Med 2009;28:3294-315. [PubMed]
- Shao W, Wang D, He J. The role of gene expression profiling in early-stage non-small cell lung cancer. J Thorac Dis 2010;2:89-99. [PubMed]
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [PubMed]
- Voon PJ, Chul Cho B, Yeo WL, et al. The role of epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of advanced stage non-small cell lung cancer. J Thorac Dis 2010;2:144-53. [PubMed]
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [PubMed]
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003;21:3016-24. [PubMed]
- Fidias P, Novello S. Strategies for prolonged therapy in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010;28:5116-23. [PubMed]
- Stinchcombe TE, Harper HD, Hensing TA, et al. The feasibility of adjuvant carboplatin and docetaxel in patients with curatively resected non-small cell lung cancer. J Thorac Oncol 2008;3:145-51. [PubMed]
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1-10. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [PubMed]
- Chhatwani L, Cabebe E, Wakelee HA. Adjuvant treatment of resected lung cancer. Proc Am Thorac Soc 2009;6:194-200. [PubMed]
- Millward MJ, Boyer MJ, Lehnert M, et al. Docetaxel and carboplatin is an active regimen in advanced non-small-cell lung cancer: a phase II study in Caucasian and Asian patients. Ann Oncol 2003;14:449-54. [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [PubMed]
- Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 2003;95:1453-61. [PubMed]
- He J, Shao W, Li S, et al. Feasibility Of Administering Adjuvant Chemotherapy Of Pemetrexed Followed By Pemetrexed/oxaliplatin Immediately Post-VATS In Patients With Completely Resected NSCLC. J Thorac Dis 2009;1:55-62. [PubMed]


