Effects of daily bathing with chlorhexidine and acquired infection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: a meta-analysis
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are typical drug resistant pathogens in healthcare settings. Critical or immunocompromised patients are at high risk for acquired infection of MRSA or VRE (1). Infection of MRSA and VRE may significantly prolong duration of hospital stay and increase the burden of in-patients. A recent review concluded that the rising morbidity of health care-associated infections (HAIs) caused by MRSA and VRE were serious problems in ICUs (2). It was reported that the prevalence of MRSA is above 60% and the nearly 30% for VRE in ICUs in the United States (3,4). Most common route of MRSA and VRE transmission is cross-transmission via contaminated hands of healthcare workers. Therefore, effective means of interrupting cross-transmission and preventing infection of MRSA and VRE is of great necessity (2).
Application of a skin antisepsis on consecutive days would reduce microbial counts (5). Chlorhexidine gluconate (CHG) has a broad-spectrum antimicrobial activity (6) and has been used widely as a hand wash and skin disinfection with good safety profile (7). Daily bathing with CHG has been reported to eradicate the colonization of high-risk pathogens including MRSA and VRE, thus decreasing the acquired risk for transmission between healthcare workers and patients (8,9). However, it is still inconclusive whether daily application with CHG bathing leads to lower acquisition of MRSA and VRE. Some articles showed that daily chlorhexidine bathing was significantly associated with the reduced acquisition of MRSA and VRE (8-10) while some other studies showed inconsistent results (11,12). Therefore, we performed this meta-analysis to investigate the association.
Materials and methods
Search strategy
An electronic search engine (PubMed), Embase and the Cochrane Central Register database were searched separately up to July 1 2013, for all eligible studies by two different reviewers (W Chen and L Li). We used the searching terms which were also MeSH terms, “chlorhexidine”, “chlorhexidine and MRSA”, “chlorhexidine and VRE”. The term “daily showering or whole body washing with chlorhexidine” was the same meaning as “daily chlorhexidine bathing”. Additional studies were identified by a hand search of references of original studies or review articles on this topic. No language restrictions were imposed. The two independent investigators (W Chen and L Li) reached consistency on all data sets for this manuscript.
Eligibility criteria
We included all the clinical trials with epidemiological study designs of retrospective surveillance, interrupted time series study, prospective interventional cohort study, before-after intervention study, and random control trial. All the literatures are published to date on the associations between the using of CHG bathing and acquired MRSA or VRE. To be included, studies had to have been published in full-articles, expressed their findings as IRR with 95% confidence interval (CI). The major study outcomes were colonization or infection of MRSA or VRE. Two independent reviewers (W Chen and L Li) examined the literatures to confirm they had fulfilled the defined inclusion criteria. Patients treated with the chlorhexidine-saturated cloth were deemed to have same effects with “daily chlorhexidine bathing”. Thus the relevant articles were also included in this review.
Data extraction
Both authors (W Chen and L Li) extracted the data independently using a data extraction form. Disagreement was settled by consensus between all authors. Information on study design, setting, study population, nature of interventions, co-interventions was collected.
Statistical analysis
Q test was used to assess the degree of heterogeneity between studies (13). If the between-study heterogeneity was not found, fixed-effect model was conducted. If I2 was ≤50%, a fixed effects model was used to calculate a pooled estimate of effect; If the I2 statistic was >50%, a random effect model was used (14). Publication bias was evaluated by the linear regression asymmetry test by Egger et al. (15). All data were analyzed in Review Manager (v.5.1.6; Oxford, England).
Results
In all, twelve studies were available in this review (8-12,16-22). Four articles were available for MRSA colonization (9,10,18,19), seven for MRSA infection (11,12,16-18,21,22), and five for VRE colonization (8-10,21,22), six for VRE infection (9,11,12,16,21,22), two for MRSA ventilator associated pneumonia (VAP) (17,18). Ten studies were interrupted time series study, two cluster-randomized trials (10,19) (Table 1).
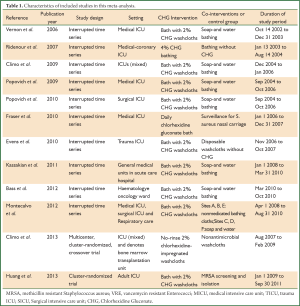
Full Table
MRSA colonization and infection
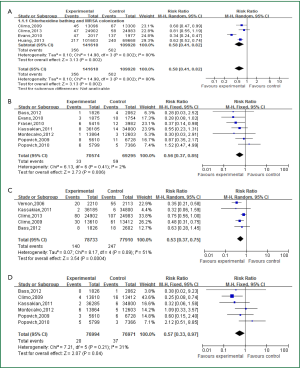
Four articles investigated the relationship between and acquired colonization of MRSA (intervention: 141,618 patient-days; control: 109,928 patient-days), all of which were performed in interrupted time series study design. As a result, daily using with CHG bathing were significantly associated with reduced colonization risk of MRSA or VRE (MRSA: IRR =0.58, 95% CI: 0.41-0.82) (Figure 1A). Interestingly, as shown in Figure 1B, the application of CHG bathing would significantly decrease acquired infection of MRSA (seven articles, intervention: 70,574 patient-days; control: 69,295 patient-days) (IRR =0.56, 95% CI: 0.37-0.85).
In subgroup analysis, we found that daily bathing with CHG significantly cut down the acquired infection of MRSA VAP (two articles, intervention: 7,290 patient-days, control: 5,736 patient-days) (IRR =0.22, 95% CI: 0.07-0.64, Pheterogeneity=0.51, I2 =0%) (Table 2). Moreover, we consistently revealed that CHG bath would decrease the acquired infection of MRSA especially in ICU settings (five articles, intervention: 32,563 patient-days, control: 32,433 patient-days; IRR =0.58, 95% CI: 0.36-0.96).
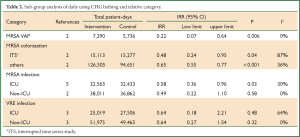
Full Table
VRE colonization and infection
Five studies were eligible to assess the impact of CHG bathing and VRE colonization (intervention: 78,733 patient-days; control: 77,370 patient-days). We found that the use of CHG bathing would significantly reduce acquired VRE colonization (IRR =0.51, 95% CI: 0.36-0.73) (Figure 1C). Meanwhile, the intervention also significantly resulted in lower acquired infection of VRE (six articles, intervention: 76,994 patient-days, control: 76,971 patient-days; IRR =0.57, 95% CI: 0.33-0.97) (Figure 1D).
Test of heterogeneity and publication bias
The test of heterogeneity related to each analysis was performed in this meta-analysis. For CHG bathing and MRSA or VRE colonization, we found that there were evidence of statistical heterogeneity (I2 =80.0% for MRSA; 51% for VRE, relatively) and random models were used for pooling of effects. However, for MRSA or VRE infections, there were no significant evidence of heterogeneity and fixed model were selected (I2 =2% for MRSA; 31% for VRE, relatively). Egger’s test was used to evaluate the potential publication bias, which was more pronounced when the higher intercept deviated from zero in linear regression analysis. No significant publication bias was found in this meta-analysis for different comparisons (all P values >0.05).
Discussion
This meta-analysis systematically reviewed the relationship between CHG bathing and the acquisition of MRSA and VRE. We identified twelve eligible articles including >250,000 patient-days, demonstrating that daily use of CHG bathing was effective in reducing the colonization of nosocomial MRSA, VRE, and significantly decreased the risks of MRSA, VRE infection.
Colonization with MRSA or VRE is a crucial risk factor for healthcare-associated infection. The bacteria can be colonized in multiple sites of the body, such as axillae, anterior naris, inguinal, perineum and so on (23,24). The strategy of decolonization of bacteria limited in single reservoir, such as mupirocin nasal ointment smearing may not enough to eradicate MRSA. Therefore, whole body bathing with CHG may be an important alternative to prevent the multi-sites’ colonization (1). Previous studies reported that CHG cleansing resulted in a persistent reduction in density of microbial skin colonization, compared with soap and water bathing (25). Vernon et al. found that the daily chlorhexidine cleaning was significantly associated with a decline in the density of VRE on patients’ skin, decreases in contamination of healthcare workers’ hands and the environment, and a decrease in the incidence of VRE colonization, compared with use of the non-medicated cloths and soap and water (8). Climo et al. performed two large multi-center clinical trials (9,10). They consistently revealed that daily chlorhexidine cleaning among ICU patients significantly reduced the acquisition of MRSA and VRE. It is plausible that the lower bacterial densities on the skin of colonized patients by the daily application of CHG bathing may have resulted in decreased rates. In this meta-analysis, we pooled all eligible studies, finding that CHG bathing significantly reduced the acquired MRSA or VRE, strongly suggesting that CHG bathing would result in reduced incidence of MRSA or VRE infection. In this meta-analysis, we proved that CHG bathing was significantly associated with 44% reduced risk of MRSA (Figure 1B) especially for ICU settings (IRR =0.58, 95% CI: 0.36-0.96) (Table 2). Moreover, we also found the incidence of VRE infection would be significantly reduced with the introduction of 2% CHG bathing (Figure 1D). It’s plausible that this CHG bathing would reduce whole bacterial burden on patient s’ skin that provided a safer environment.
Evens et al. performed a retrospective analysis of data collected 6 months before and after institution of CHG bathing protocol, firstly reported that in critically ill trauma patients, who used of the same chlorhexidine washcloths resulted in decreased incidence of VAP (18). Interestingly, in the subgroup analysis, CHG bathing would cut down 78% risk of MRSA VAP (Table 2). And large well-designed random clinical trial was warrant to explore this association.
However, there was no evidence of inducing chlorhexidine resistance in the susceptibility test of bacterial isolates (26). Several previous published articles reported that chlorhexidine resistance was rare among both staphylococci and enterococci with reported minimum inhibitory concentrations (MICs) to chlorhexidine (staphylococci: 0.2-3 mg/L) and (enterococci: 1-6 mg/L) (27-29). There was no any evidence of resistance to chlorhexidine among MRSA and VRE isolates (8,20,30). Remarkably, Batra et al. reported that daily chlorhexidine bathing was associated with a highly significant, immediate 70% reduction in acquisition of non-TW MRSA strains (RR =0.3, 95 % CI: 0.19-0.47) whereas shown an increase in acquisition of TW MRSA strains (RR =3.85, 95 % CI: 0.80-18.59). They genotyped the isolations, finding that all TW MRSA strains (sequence type 239) (21 of 21 isolates) and 5% (1 of 21 isolates) of non-TW MRSA strains tested carried the chlorhexidine resistance loci qacA/B. Meanwhile, they also found that in vitro chlorhexidine minimum bactericidal concentrations (MBC) of TW strains were 3-fold higher than those of non-TW MRSA strains. Both of them could account for the different effects to chlorhexidine between TW MRSA and Non-TW MRSA.
In conclusion, we found that CHG bathing would result in the decreased acquired infection of MRSA or VRE. If this intervention is widely implemented in clinical practice, vigilance for emerging resistance should be required.
Acknowledgements
Disclosure: This study was supported by grants from Jiangsu Province Projects of preventive medicine research (Y2012046) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and (JX10231801). The authors declare no conflict of interest.
References
- Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335-44. [PubMed]
- Huskins WC. Interventions to prevent transmission of antimicrobial-resistant bacteria in the intensive care unit. Curr Opin Crit Care 2007;13:572-7. [PubMed]
- Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin Infect Dis 2006;42:389-91. [PubMed]
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470-85. [PubMed]
- Webster J, Osborne S. Meta-analysis of preoperative antiseptic bathing in the prevention of surgical site infection. Br J Surg 2006;93:1335-41. [PubMed]
- Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis 2008;46:274-81. [PubMed]
- Rosenberg A, Alatary SD, Peterson AF. Safety and efficacy of the antiseptic chlorhexidine gluconate. Surg Gynecol Obstet 1976;143:789-92. [PubMed]
- Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306-12. [PubMed]
- Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858-65. [PubMed]
- Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 2013;368:533-42. [PubMed]
- Popovich KJ, Hota B, Hayes R, et al. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med 2010;36:854-8. [PubMed]
- Popovich KJ, Hota B, Hayes R, et al. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol 2009;30:959-63. [PubMed]
- Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323:157-62. [PubMed]
- Karki S, Cheng AC. Impact of non-rinse skin cleansing with chlorhexidine gluconate on prevention of healthcare-associated infections and colonization with multi-resistant organisms: a systematic review. J Hosp Infect 2012;82:71-84. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med 2012;125:505-11. [PubMed]
- Fraser TG, Fatica C, Scarpelli M, et al. Decrease in Staphylococcus aureus colonization and hospital-acquired infection in a medical intensive care unit after institution of an active surveillance and decolonization program. Infect Control Hosp Epidemiol 2010;31:779-83. [PubMed]
- Evans HL, Dellit TH, Chan J, et al. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch Surg 2010;145:240-6. [PubMed]
- Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255-65. [PubMed]
- Ridenour G, Lampen R, Federspiel J, et al. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol 2007;28:1155-61. [PubMed]
- Bass P, Karki S, Rhodes D, et al. Impact of chlorhexidine-impregnated washcloths on reducing incidence of vancomycin-resistant enterococci colonization in hematology-oncology patients. Am J Infect Control 2013;41:345-8. [PubMed]
- Kassakian SZ, Mermel LA, Jefferson JA, et al. Impact of chlorhexidine bathing on hospital-acquired infections among general medical patients. Infect Control Hosp Epidemiol 2011;32:238-43. [PubMed]
- Lautenbach E, Nachamkin I, Hu B, et al. Surveillance cultures for detection of methicillin-resistant Staphylococcus aureus: diagnostic yield of anatomic sites and comparison of provider- and patient-collected samples. Infect Control Hosp Epidemiol 2009;30:380-2. [PubMed]
- Marshall C, Spelman D. Re: is throat screening necessary to detect methicillin-resistant Staphylococcus aureus colonization in patients upon admission to an intensive care unit? J Clin Microbiol 2007;45:3855. [PubMed]
- Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073-9. [PubMed]
- Ho KM, Litton E. Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. J Antimicrob Chemother 2006;58:281-7. [PubMed]
- Penna TC, Mazzola PG, Silva Martins AM. The efficacy of chemical agents in cleaning and disinfection programs. BMC Infect Dis 2001;1:16. [PubMed]
- Russell AD. Bacterial resistance to disinfectants: present knowledge and future problems. J Hosp Infect 1999;43:S57-68. [PubMed]
- Suller MT, Russell AD. Antibiotic and biocide resistance in methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus. J Hosp Infect 1999;43:281-91. [PubMed]
- Batra R, Cooper BS, Whiteley C, et al. Efficacy and limitation of a chlorhexidine-based decolonization strategy in preventing transmission of methicillin-resistant Staphylococcus aureus in an intensive care unit. Clin Infect Dis 2010;50:210-7. [PubMed]


