Chronic obstructive pulmonary disease in China: the potential role of indacaterol
Chronic obstructive pulmonary disease (COPD) is a disease characterized by persistent airflow limitation that is usually progressive and not fully reversible (1). The burden of the disease is high as it leads to disability and impairs quality of life (2), in addition to having a high impact on health care costs (3). Dyspnea, chest discomfort, chronic cough, and sputum production are the characteristic symptoms of COPD (1). It is usually seen that COPD patients initially seek a physician’s advice after encountering breathing problems, even though cough is the first symptom that appears in these patients and often goes unrecognized. By the time the patient consults the physician, the condition is already aggravated (4). In addition, COPD is quite often under-diagnosed by physicians because of lack of diagnostic skills, delay in diagnostic testing (5,6), and underestimation of the symptom severity (7). Patients can also be reluctant to seek medical treatment, which further contributes toward the under-diagnosis. All these factors together make COPD a leading cause of morbidity and mortality world-wide (8). Without adequate diagnosis, continued exposure to the risk factors for COPD, including tobacco smoke, air pollution, smoke from biofuels, and occupational dusts and chemicals, contributes to the worsening of the disease (1). Additionally, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy document for the pharmacological management of COPD recommends an individualized step-up, or treatment intensification approach, when the management of symptoms is not satisfactory; this requires continuous close monitoring of patients, and access to a wide range of medications (1).
Epidemiology of COPD in China
Prevalence
In China, COPD is increasingly becoming a cause of public health concern and ranks first among the causes of disability (9). According to an estimation, the overall prevalence rate of COPD in China is 8.2% (10) and mortality rate because of the disease is approximately 1.6% (11). COPD is reported to be the fourth leading cause of death in rural areas, with a prevalence rate of 4.4% to 16.7%. In urban areas it is considered to be the third leading cause of death, with a prevalence rate of 6.7% to 8.3% (12,13). Additionally, men are more likely to encounter COPD than women (8.3% to 18.9% men versus 3.8% to 7.1% women) (13). The major risk factors accounted for COPD in China are tobacco smoking, use of biomass fuels, and genetic susceptibility. Apart from these, the socioeconomic status of an individual, low education levels, malnutrition, air pollution, respiratory infections, and low physical activity contribute toward an increased risk of COPD (13).
Economic burden
The treatment of COPD in China has high financial implications for the patient. A survey conducted in 2006 on 723 COPD outpatients in 6 major Chinese cities found that the average annual direct medical cost (including outpatient, inpatient, and medicine costs) was 11,744 RMB Yuan (approximately $1,732) whereas the direct nonmedical cost (including transportation, nutrition, and nursing costs) was 1,570 RMB Yuan (approximately $232) for every urban patient. The total expenditure per COPD patient accounted for 40% of an average family’s income (14).
Another survey was conducted in 2008 on 7,383 COPD patients in rural areas of China. The study estimated the direct economic burden of the disease to be 1,090 RMB Yuan (approximately $173) per person. This was mainly driven by the cost of outpatient services (42.3%), hospitalization (32.5%), and self-financed medicines (20.5%). The indirect economic burden of COPD was estimated to be 20,605 RMB Yuan (approximately $3,276) per person. The overall economic burden of COPD accounted for nearly one-third of an average family’s income in rural areas of China (15).
Treatment of COPD in China
The national strategy for COPD management in China encourages physicians to adopt the GOLD recommendations for the management of the disease (16). However, proper implementation of the guidelines is lacking. The diagnosis of COPD is usually delayed in China because of a lack of physician awareness about biomarkers, symptoms, and risk factors (17,18). Only 20% of primary care physicians are fully knowledgeable about the pharmacological recommendations for COPD included in the GOLD document (19). In China, 64.7% patients with COPD show at least one of the respiratory symptoms (cough, phlegm, wheezing, and breathlessness), whereas 35.3% patients with COPD are found to be asymptomatic (10). Most physicians in China diagnose COPD without evaluating the severity with spirometry, although it is considered to be required for confirmation of the diagnosis according to the GOLD recommendations (14). As a result, less than one-third of COPD diagnoses in China involve the use of spirometry (14). It is observed that the medications prescribed by physicians often differ from the GOLD strategy recommendations, and the most often prescribed medications are expectorants, followed by β2-agonists (albuterol) and anticholinergics (ipratropium) (20). Furthermore, short-acting bronchodilators are frequently prescribed, rather than long-acting bronchodilators (12).
Long-acting β2-agonists (LABAs) as treatment option for COPD
According to the GOLD 2013 strategy document, bronchodilators are central to symptom management in COPD. Long-acting bronchodilators including LABAs and long-acting muscarinic antagonists (LAMAs) are both more convenient and effective at producing maintained symptom relief than short-acting bronchodilators. Hence, they are recommended as the first-line maintenance treatment for patients who are more symptomatic. Inhaled corticosteroids (ICSs) are reserved for patients at increased risk of exacerbations, either due to severe or very severe airflow obstruction (1). Two twice-daily LABAs (salmeterol and formoterol) have been commercially available since the 1980s for the treatment of COPD. Indacaterol is the first once-daily LABA to be approved, providing 24-h bronchodilation, and a fast onset of action following the first dose (21,22).
Mechanism of action of LABAs
The pharmacological pathway following LABA administration suggests that on inhalation the drug deposits on the bronchial mucosa of the lungs. It dissolves and diffuses through the cell components of the bronchial tissue, ultimately reaching the bronchial smooth muscles (23). Here the β2-agonists act by causing relaxation of the smooth muscles, leading to bronchodilation. Smooth muscle relaxation is because of β2-adrenoreceptor-mediated activation of adenylate cyclase, which in turn increases the concentration of intracellular cyclic adenosine monophosphate (24). It is hypothesized that the prolonged duration of action of indacaterol could be explained by the higher partitioning of indacaterol (compared with salmeterol) into lipid raft microdomains, which are highly ordered membrane microdomains encompassing the β2-adrenoceptors. Further, the faster onset of action of indacaterol compared with that of salmeterol could be explained by the higher intrinsic efficacy of indacaterol compared with that of salmeterol (25).
Salmeterol and formoterol have been shown to significantly improve forced expiratory volume in 1 sec (FEV1), dyspnea, health-related quality of life, and exacerbation rates compared with placebo (26-31), but they have no effect on mortality and rate of decline of lung function. Salmeterol has also been shown to reduce the rate of hospitalization (27).
Unmet needs of COPD
COPD is a “life-limiting illness”, which is defined as an illness that shortens an individual’s lifespan and likely leads to death (32,33). The severe stages of the disease (especially GOLD Group D) are associated with worsening of airflow limitation, greater shortness of breath, fatigue, reduction in exercise capacity and frequent episodes of exacerbations (1). All these events ultimately result in a deterioration in health-related quality of life (34,35). Studies have shown that approximately 60% of patients with COPD face difficulties in their daily activities, with 75% having difficulty in climbing stairs and 45% being unable to work (36).
Despite the high burden of symptoms, patients with COPD have various unmet needs-the major ones being an effective diagnosis, and primary prevention measures. Of course, the most effective preventative measure is to avoid exposure to the causative factors-mainly tobacco smoking and exposure to biomass fuels (37). Smoking cessation is one of the few interventions that have been shown to delay or prevent COPD disease progression, with both bupropion and varenicline having been shown to increase the rate of smoking cessation (38,39). Better ventilation can be another way of prevention as shown in a study in China (40). Another unmet need is better symptom control of COPD and fewer incidences of exacerbations. This can be achieved by using appropriate bronchodilators (41) or ICSs (27), pulmonary rehabilitation (42), and oxygen therapy (43). COPD is also associated with increased rates of comorbidities such as cardiovascular diseases (44), osteoporosis (45), diabetes (46), pneumonia (47), anxiety, and depression (48). A better life expectancy with less comorbidity therefore is key unmet patient needs.
Indacaterol—once-daily LABA
Indacaterol 150 µg was approved in China on June 15, 2012, as once-daily LABA for the maintenance treatment of airflow limitation in patients with COPD. In this review we will discuss the results of lung function, dyspnea, rescue medication, exacerbations, St George’s respiratory questionnaire (SGRQ), and daytime and nighttime symptoms, from indacaterol studies of 12-week duration or longer that included the 150 µg dose. In addition, results from two studies of indacaterol plus tiotropium will be summarized. The study designs of the 10 applicable studies are summarized in Table 1.
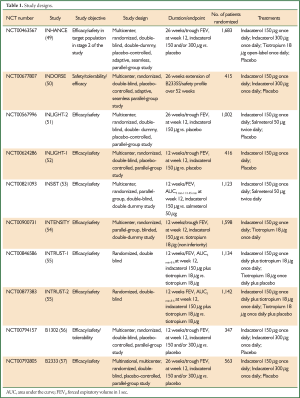
Full Table
Lung function
Compared with placebo, the effect of indacaterol 150 μg on trough FEV1 (measured 24-h post dose) after 12 weeks of treatment, was found to be in the range of 0.13-0.18 L, differences that were both statistically significant and clinically relevant (P<0.001), supporting the suitability of indacaterol for once-daily dosing (Table 2) (49-52,56,57). After 26 weeks of treatment the difference versus placebo in trough FEV1 with indacaterol 150 μg was statistically significant and clinically meaningful, and similar (0.13-0.18 L, P<0.001) to that at week 12 (49-51,57). In the INDORSE study, indacaterol 150 μg showed significant bronchodilation versus placebo at week 52, with the between-treatment difference being 0.17 L (P<0.001) (50).

Full Table
In the 12-week INTENSITY study, indacaterol 150 μg when compared with blinded tiotropium 18 μg showed similar efficacy on trough FEV1 after 12 weeks of treatment (1.44 versus 1.43 L, respectively), with the treatment difference meeting the predefined criteria for non-inferiority [0 L, 95% confidence interval (CI): –0.02, 0.02; P<0.001] (Figure 1) (54). In the INHANCE study with open-label tiotropium, indacaterol 150 μg showed a 0.04-0.05 L treatment difference versus tiotropium in 24-h post dose trough FEV1 at week 12 (49). This treatment difference was significant when tested for superiority (P≤0.01) and non-inferiority (P<0.001) (49).
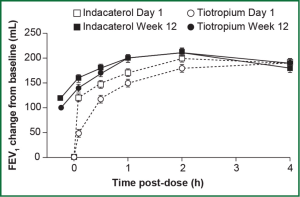
Indacaterol also showed statistically superior bronchodilation compared with salmeterol 50 μg twice daily in terms of trough FEV1 at week 12 in the INLIGHT-2 and INSIST studies [least squares mean (LSM) differences 0.06 and 0.06 L, respectively; P<0.001] (51,53) and at week 26 in INLIGHT-2 (LSM difference 0.07 L; P<0.001) (53). Results of FEV1 standardized area under the curve (AUC) measured from 5 min to 11 h 45 min at week 12 (the primary endpoint of INSIST) also demonstrated superiority of indacaterol over salmeterol (LSM difference 0.057 L, 95% CI: 0.035-0.079; P<0.001) (53).
Dyspnea
One of the main disabling symptoms of COPD is breathlessness (58), which can lead to limitations in lifestyle. Transition dyspnea index (TDI) is a scale that measures dyspnea by measuring the breathlessness related to daily activities and compares it over time or in response to other treatments (59).
Indacaterol 150 μg was associated with statistically significantly greater TDI scores than placebo at weeks 12 and 26 (P<0.001 in the INHANCE and INLIGHT-2 studies; P<0.01 in the B2333 study) (49,51,57). In the B1302 study the indacaterol versus placebo difference was statistically significant at week 12 (LSM difference 1.30; P≤0.001) (56). Compared with blinded tiotropium 18 μg, in INTENSITY a LSM difference of 0.58 was observed with indacaterol 150 μg at week 12, which was statistically significant (P<0.001) (54). However, in INHANCE the TDI score in the indacaterol 150 μg group was numerically higher than that in the open-label tiotropium 18 μg, although this difference did not reach statistical significance at either week 12 or week 26 (49). The TDI total score with indacaterol 150 μg was also statistically significant compared with salmeterol 50 μg (twice daily) at week 12 in both INSIST and INLIGHT-2, with adjusted mean differences of 0.63 (P<0.001) and 0.55 (P=0.015), respectively; the difference at week 26 in INLIGHT-2 was not statistically significant (51,53).
Compared with placebo, a significant proportion of patients taking indacaterol 150 μg achieved a clinically important improvement from baseline (≥1 point) (60) in TDI total score at week 12 (P<0.05) (49,51,56,57) and week 26 (P<0.05) (49,51,57). A significantly higher proportion of patients taking indacaterol 150 μg achieved a clinically meaningful improvement in TDI total score compared with those taking blinded tiotropium in INTENSITY [odds ratio (OR) 1.49; P<0.001] and salmeterol in INSIST (OR 1.41; P<0.05) (53,54). This improvement in dyspnea can be partly explained by the reduced hyperinflation, which allows the patients to be more physically active, thereby improving their health status (61,62). As dyspnea is an important clinical indicator of COPD, an improvement in TDI score indicates indacaterol to be beneficial in controlling the symptoms of COPD.
Rescue medication
In all of these studies, patients recorded their use of rescue medication twice daily in a diary. The mean daily number of puffs of rescue medication was statistically significantly lower (P≤0.001) for indacaterol 150 µg compared with that for placebo and tiotropium 18 µg (both open label and blinded) during the course of over 12- (52,54), 26- (49,51,57), and 52-week (50) treatment periods (Table 3). Compared with salmeterol 50 µg twice daily, patients taking indacaterol 150 µg used fewer puffs of rescue medication per day. The change from baseline in mean daily number of puffs of rescue medication was numerically greater in the indacaterol group than in the salmeterol group in the INLIGHT-2 study (LSM ± standard error: –1.3±0.16 and –1.2±0.16, for indacaterol and salmeterol, respectively) (51). The difference reached statistical significance in the INSIST study (LSM difference: –0.18; P<0.05) (53).
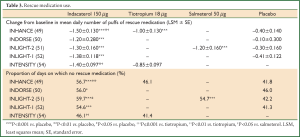
Full Table
The proportion of days with no rescue medication use was significantly increased (P<0.05) for indacaterol 150 μg compared with that for placebo, tiotropium 18 µg (both open label and blinded), and salmeterol 50 µg (49-54,57).
Exacerbations
None of these studies were designed to evaluate the effect of treatments on exacerbations, and the rates of exacerbations in all treatment groups were low. In the INHANCE study, the proportion of patients experiencing exacerbations in the indacaterol 150 µg group (17.3%) was found to be numerically lower than that in the placebo (21.8%) and open-label tiotropium 18 µg groups (19.0%) after 6 months of study (49). The analysis of time to first COPD exacerbation showed a statistically reduced risk for indacaterol compared with that for placebo (hazard ratio 0.69; 95% CI: 0.51-0.94; P=0.019). The annual rate of COPD exacerbations (calculated as total number of exacerbations/total number of treatment years) was significantly lower in the indacaterol 150 µg group compared with that in the placebo group (rate ratio 0.64-0.67; P<0.05) (49,50). These data suggest that the sustained bronchodilator effect of indacaterol is associated with reduction in the rate of COPD exacerbations.
SGRQ
Indacaterol 150 µg showed a statistically significant improvement in the SGRQ score compared with placebo at week 12 (LSM difference –2.8 to –6.3 units; P<0.01) and week 26 (LSM difference –3.3 to –5.0 units; P≤0.001) (49,51,56). The score was also statistically significant when compared with that for tiotropium 18 µg (both open label and blinded) at week 12 (P<0.05) (49,54) and compared with open-label tiotropium 18 µg at week 26 (P≤0.01) (49). However, with salmeterol 50 µg the difference was significant only at week 12 (P<0.05) in INLIGHT-2 (health status was not assessed in INSIST) (51).
The proportion of patients with a clinically important improvement (≥4 units) in the SGRQ total score (63) was significantly higher for indacaterol 150 µg compared with that for placebo at week 12 (OR 1.40-2.41; P<0.05) and week 26 (OR 1.75-1.96; P<0.001) (49,51). However, in another study the proportion of patients achieving the clinically important difference did not reach statistical significance versus placebo, perhaps due to a large improvement in the placebo group (OR 1.74; P=0.067) (56). A significantly higher number of patients taking indacaterol 150 µg achieved the clinically important difference compared to those taking blinded tiotropium 18 µg at Week 12 in INTENSITY (P<0.001) (54), compared to those taking open-label tiotropium 18 µg at Week 26 in INHANCE (P≤0.01) (49) and compared to those taking salmeterol 50 µg at Week 12 in INLIGHT-2 (P<0.01) (51).
Daytime and nighttime symptoms
Symptoms were recorded in all these studies using a patient diary. Indacaterol 150 µg showed significant improvements as compared with placebo in the proportion of nights with no nighttime awakenings (P<0.05) (49,51,56,57), days with no daytime symptoms (P<0.05) (49,51), and days able to perform usual activities (P<0.01) (49,51,56,57). Compared with salmeterol 50 µg twice daily, indacaterol showed significant improvements in the percentage of days able to perform usual activities (P<0.05) (51). When compared with tiotropium 18 µg (both open label and blinded), indacaterol showed numerical improvements in the percentage of days with no daytime symptoms, nights with no awakenings, and days able to undertake usual activities (49,54). These results suggest that indacaterol 150 µg has an important role in COPD symptom management.
INTRUST 1 and INTRUST 2 studies
The results from all the above-mentioned studies demonstrate the efficacy profile of indacaterol 150 μg as monotherapy in patients with COPD. Given the recommendations in the GOLD strategy document that in patients with symptoms not adequately managed with a single bronchodilator, the combination of a LABA and a LAMA could be considered, two matching studies (INTRUST 1 and INTRUST 2) were conducted to compare the efficacy of indacaterol 150 μg plus tiotropium 18 μg with that of tiotropium 18 μg alone.
It was found that the concurrent administration of indacaterol plus tiotropium showed superior bronchodilation (P<0.001) compared with that of tiotropium alone in terms of FEV1 AUC5 min-8 h following 12 weeks of treatment, with the between treatment difference being 0.13 L (95% CI: 0.1-0.15) and 0.12 L (95% CI: 0.09-0.14) in INTRUST 1 and INTRUST 2, respectively.
Similarly, indacaterol plus tiotropium performed better than tiotropium alone in terms of trough FEV1 (last observation carried forward) at week 12 with differences of 0.08 L (95% CI: 0.05-0.1; P<0.001) and 0.07 L (95% CI: 0.05-0.09; P<0.001) in INTRUST 1 and INTRUST 2, respectively. The two studies, in addition, showed significant (P<0.05) lung deflation (increased inspiratory capacity) after 12 weeks of treatment with indacaterol plus tiotropium as compared with tiotropium alone (Figure 2) (55).
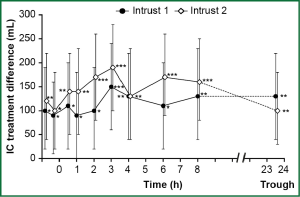
Safety
Indacaterol 150 µg was associated with an overall good safety and tolerability profile. We present the pooled data from clinical studies of 3- to 12-month duration in patients with moderate-to-severe COPD (double-blind indacaterol 150 µg once daily, n=2,611; open-label or blinded tiotropium 18 µg once daily, n=1,214; salmeterol 50 µg twice daily, n=895; placebo, n=2,012). The most common adverse events (AEs) with indacaterol 150 µg were COPD worsening, nasopharyngitis, headache, cough, and upper urinary tract infection (Table 4). Most of the AEs were mild or moderate in severity, and the incidence rate was generally similar in all groups. AEs commonly associated with β2-agonist class effects are considered to be insomnia, anxiety, tremor, palpitation, and tachycardia (Table 4). Overall, the incidence rate of severe AEs (SAEs) was similar (or numerically lower) with indacaterol 150 µg than with placebo. The most common SAE with indacaterol 150 µg was COPD worsening (0.05 per patient year). The rate of respiratory SAEs (leading to hospitalization, intubation, or death) was not significantly increased with any of the active treatments compared with placebo. COPD exacerbation rates were significantly reduced with indacaterol 150 µg versus placebo. Hazard ratios versus placebo for major cardiovascular AEs was <1 for indacaterol 150 µg (64).
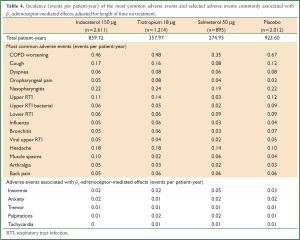
Full Table
A study done in predominantly Chinese patients with COPD reported that the overall incidence of AEs was comparable between indacaterol 150 µg (49.2%) and placebo groups (45.2%) (57). Ventricular extrasystole was the most common AE that was suspected to be related to the study drug (2.1% and 2.2% in indacaterol 150 µg and placebo groups, respectively). This was followed by cough with a frequency of 1.1% and 0.5% in the two groups, respectively. The overall incidence of SAEs was comparable between the treatment groups, with COPD exacerbations being the most common SAE (3.2% and 3.8% in indacaterol 150 µg and placebo groups, respectively) (57).
Summary
COPD is a widely prevalent disease in China, with smoking and biomass fuels being the major causes of the disease. Additionally, the economic burden of the disease is high in both rural and urban areas of China. The diagnosis and management of the disease generally is not in accordance with the GOLD recommendations, with short-acting bronchodilators being frequently prescribed by physicians, despite long-acting agents being the recommended treatment option for patients with all but infrequent symptoms. The LABAs previously available were salmeterol and formoterol, which have a twice-daily dosing regimen; indacaterol is a once-daily LABA that has been recently introduced and approved at a dose of 150 µg once daily in China. Various phase III studies have reported the efficacy and safety of this dose of indacaterol over the already existing bronchodilators, with improvements in bronchodilation, breathlessness, health status, and rescue medication use in studies of up to 26 weeks (49-52,56,57). Indacaterol 150 µg also shows a good overall safety and tolerability profile as compared with placebo and other available bronchodilators (64). Hence, once-daily indacaterol 150 µg could be considered as an effective treatment option in China for patients with COPD.
Acknowledgements
The authors were assisted in the writing of this review manuscript by Tania Peshin, a professional medical writer (Novartis).
Disclosure: Jinming Gao was the recipient of several grants from the Natural Sciences Foundation of China (No. 81170040), National Science and Technology Pillar Program during the Twelfth five-year Period (No. 2012BAI05B00) and the Peking Union Medical College. Niyati Prasad is an employee of Novartis.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2013.
- Ferrer M, Alonso J, Morera J, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med 1997;127:1072-9. [PubMed]
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117:5S-9S. [PubMed]
- den Otter JJ, van DB, van Schayck CP, et al. How to avoid underdiagnosed asthma/chronic obstructive pulmonary disease? J Asthma 1998;35:381-7. [PubMed]
- Bye MR, Kerstein D, Barsh E. The importance of spirometry in the assessment of childhood asthma. Am J Dis Child 1992;146:977-8. [PubMed]
- Joyce DP, Chapman KR, Kesten S. Prior diagnosis and treatment of patients with normal results of methacholine challenge and unexplained respiratory symptoms. Chest 1996;109:697-701. [PubMed]
- Jones K. Asthma care in general practice--time for revolution? Br J Gen Pract 1991;41:224-6. [PubMed]
- Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet 2003;362:1053-61. [PubMed]
- Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nat Med 1998;4:1241-3. [PubMed]
- Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753-60. [PubMed]
- Fang XC, Wang XD, Bai CX. Burden and importance of proper management about chronic obstructive pulmonary disease in China. Int J Respir 2011;31:493-7.
- Respiratory Diseases Branch of Chinese Medical Association. A national guideline for the diagnosis, management, and prevention of chronic obstructive pulmonary disease in China. Chin J Tuberc Respir Dis 2007;30:8-17.
- Fang X, Wang X, Bai C. COPD in China: the burden and importance of proper management. Chest 2011;139:920-9. [PubMed]
- He QY, Zhou X, Xie CM, et al. Impact of chronic obstructive pulmonary disease on quality of life and economic burden in Chinese urban areas. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:253-7. [PubMed]
- Lou P, Zhu Y, Chen P, et al. Vulnerability, beliefs, treatments and economic burden of chronic obstructive pulmonary disease in rural areas in China: a cross-sectional study. BMC Public Health 2012;12:287. [PubMed]
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55. [PubMed]
- Li ZP, Huang JQ, Tang KJ. Retrospective studies on 713 cases chronic obstructive pulmonary disease. Zhonghua Liu Xing Bing Xue Za Zhi 2003;24:722-4. [PubMed]
- Shen N, Yao WZ, Zhu H. Patient’s perspective of chronic obstructive pulmonary disease in Yanqing county of Beijing. Zhonghua Jie He He Hu Xi Za Zhi 2008;31:206-8. [PubMed]
- Zhang RB, He QY. Awareness of knowledge of COPD by doctors in district and community hospitals. Chin J Prev Control Chronic Dis 2009;17:61-3.
- He QY, Zhou X, Xie CM, et al. The investigation of the treatment conditions in stable COPD patients in partial cities in China. Chin J Practic Intern Med 2009;29:354-7.
- Hosoe M, Woessner R, Matsushima S, et al. Efficacy, safety and pharmacokinetics of indacaterol in Caucasian and Japanese patients with chronic obstructive pulmonary disease: a comparison of data from two randomized, placebo-controlled studies. Clin Drug Investig 2011;31:247-55. [PubMed]
- Vogelmeier C, Ramos-Barbon D, Jack D, et al. Indacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropium. Respir Res 2010;11:135. [PubMed]
- Lötvall J. The long and short of beta2-agonists. Pulm Pharmacol Ther 2002;15:497-501. [PubMed]
- Lulich KM, Goldie RG, Paterson JW. Beta-adrenoceptor function in asthmatic bronchial smooth muscle. Gen Pharmacol 1988;19:307-11. [PubMed]
- Lombardi D, Cuenoud B, Kramer SD. Lipid membrane interactions of indacaterol and salmeterol: do they influence their pharmacological properties? Eur J Pharm Sci 2009;38:533-47. [PubMed]
- Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J 1997;10:815-21. [PubMed]
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89. [PubMed]
- Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med 1995;89:357-62. [PubMed]
- Rossi A, Kristufek P, Levine BE, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest 2002;121:1058-69. [PubMed]
- Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res 2010;11:149. [PubMed]
- Ulrik CS. Efficacy of inhaled salmeterol in the management of smokers with chronic obstructive pulmonary disease: a single centre randomised, double blind, placebo controlled, crossover study. Thorax 1995;50:750-4. [PubMed]
- Clayton JM, Hancock KM, Butow PN, et al. Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. Med J Aust 2007;186:S77,S79,S83-108.
- Steinhauser KE, Arnold RM, Olsen MK, et al. Comparing three life-limiting diseases: does diagnosis matter or is sick, sick? J Pain Symptom Manage 2011;42:331-41. [PubMed]
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. [PubMed]
- Spencer S, Calverley PM, Sherwood BP, et al. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:122-8. [PubMed]
- Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J 2002;20:799-805. [PubMed]
- Yin P, Jiang CQ, Cheng KK, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet 2007;370:751-7. [PubMed]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47-55. [PubMed]
- Tashkin D, Kanner R, Bailey W, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet 2001;357:1571-5. [PubMed]
- Chapman RS, He X, Blair AE, et al. Improvement in household stoves and risk of chronic obstructive pulmonary disease in Xuanwei, China: retrospective cohort study. BMJ 2005;331:1050. [PubMed]
- Scott S, Walker P, Calverley PM. COPD exacerbations. 4: Prevention. Thorax 2006;61:440-7. [PubMed]
- Ries AL, Kaplan RM, Myers R, et al. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med 2003;167:880-8. [PubMed]
- O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:892-8. [PubMed]
- Calverley PM, Anderson JA, Celli B, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010;65:719-25. [PubMed]
- Silva DR, Coelho AC, Dumke A, et al. Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care 2011;56:961-8. [PubMed]
- Baker EH, Bell D. Blood glucose: of emerging importance in COPD exacerbations. Thorax 2009;64:830-2. [PubMed]
- Obert J, Burgel PR. Pneumococcal infections: association with asthma and COPD. Med Mal Infect 2012;42:188-92. [PubMed]
- Lewis KE, Annandale JA, Sykes RN, et al. Prevalence of anxiety and depression in patients with severe COPD: similar high levels with and without LTOT. COPD 2007;4:305-12. [PubMed]
- Donohue JF, Fogarty C, Lotvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010;182:155-62. [PubMed]
- Chapman KR, Rennard SI, Dogra A, et al. Long-term safety and efficacy of indacaterol, a long-acting beta(2)-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest 2011;140:68-75. [PubMed]
- Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011;37:273-9. [PubMed]
- Feldman G, Siler T, Prasad N, et al. Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med 2010;10:11. [PubMed]
- Korn S, Kerwin E, Atis S, et al. Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med 2011;105:719-26. [PubMed]
- Buhl R, Dunn LJ, Disdier C, et al. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J 2011;38:797-803. [PubMed]
- Mahler DA, D’Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax 2012;67:781-8. [PubMed]
- Kinoshita M, Lee SH, Hang LW, et al. Efficacy and safety of indacaterol 150 and 300 microg in chronic obstructive pulmonary disease patients from six Asian areas including Japan: a 12-week, placebo-controlled study. Respirology 2012;17:379-89. [PubMed]
- Yao W, Wang C, Zhong N, et al. Effect of once-daily indacaterol in a predominantly Chinese COPD population: a 26-week Asia-Pacific study. Eur Respir J 2012;40:2107.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [PubMed]
- Mahler DA. Mechanisms and measurement of dyspnea in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:234-8. [PubMed]
- Witek TJ Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 2003;21:267-72. [PubMed]
- Cooper CB. Airflow obstruction and exercise. Respir Med 2009;103:325-34. [PubMed]
- Hanania NA, Donohue JF. Pharmacologic interventions in chronic obstructive pulmonary disease: bronchodilators. Proc Am Thorac Soc 2007;4:526-34. [PubMed]
- Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD 2005;2:75-9. [PubMed]
- Donohue JF, Singh D, Kornmann O, et al. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis 2011;6:477-92. [PubMed]


